
Abstract
Aims: The aim of this study was to estimate the cost-effectiveness of transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) in patients at lower surgical risk.
Methods and results: Discounted costs from a societal perspective and effectiveness as quality-adjusted life years (QALYs) were projected to lifetime via a decision-analytic model calibrated to 60-month data from the NOTION trial. The base case assumed a scenario in which any mortality benefit would gradually fade out over time, with other scenarios explored in sensitivity analyses. The incremental cost-effectiveness ratio (ICER) was compared to the country-specific willingness-to-pay (WTP) threshold of 1.13 million Danish kroner (DKK). The base case ICER was DKK 696,264/QALY (around €72,100/QALY via purchasing parity adjustment). Variation in long-term mortality beyond five years led to limited variation of incremental costs (DKK 64,200 to 64,600), but a more pronounced variation in incremental QALYs (0.07 to 0.19 QALYs for most conservative and optimistic assumptions, compared to base case of 0.09 QALYs). All resulting ICERs (range DKK 334,200 to DKK 904,100 per QALY gained) were below the WTP threshold.
Conclusions: TAVI in a cohort of primarily low surgical risk patients was found to be a cost-effective treatment strategy in the Danish healthcare system. Cost-effectiveness analyses in other settings are warranted as are registries given the sensitivity of the model to long-term mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) has experienced a remarkable uptake in patients with severe aortic stenosis and high surgical risk, with over 54,000 procedures between 2012 and 2015 in the USA alone1. Its cost-effectiveness in these populations has been established in various settings and for both balloon-expandable and self-expanding TAVI devices2.
The Nordic Aortic Valve Intervention (NOTION) trial was the first trial in patients with severe aortic stenosis and lower surgical risk randomised to TAVI or surgical bioprosthetic aortic valve replacement (SAVR)3,4; four-year results were recently presented (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017). The NOTION trial demonstrated no significant difference in the primary outcome, a composite of all-cause death, stroke, or myocardial infarction (MI), between TAVI and SAVR. Non-significant differences persisted after 244 and 48 months of follow-up, respectively (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017).
Given that surgical candidates who are not at increased surgical risk might have fewer (or no) incremental benefits from TAVI compared to SAVR but potentially higher costs, the “real-world” value of TAVI outside carefully selected high or extreme surgical risk patient cohorts has been uncertain. The objective of the present study was therefore to estimate the long-term cost-effectiveness of TAVI compared to SAVR in the NOTION cohort.
Methods
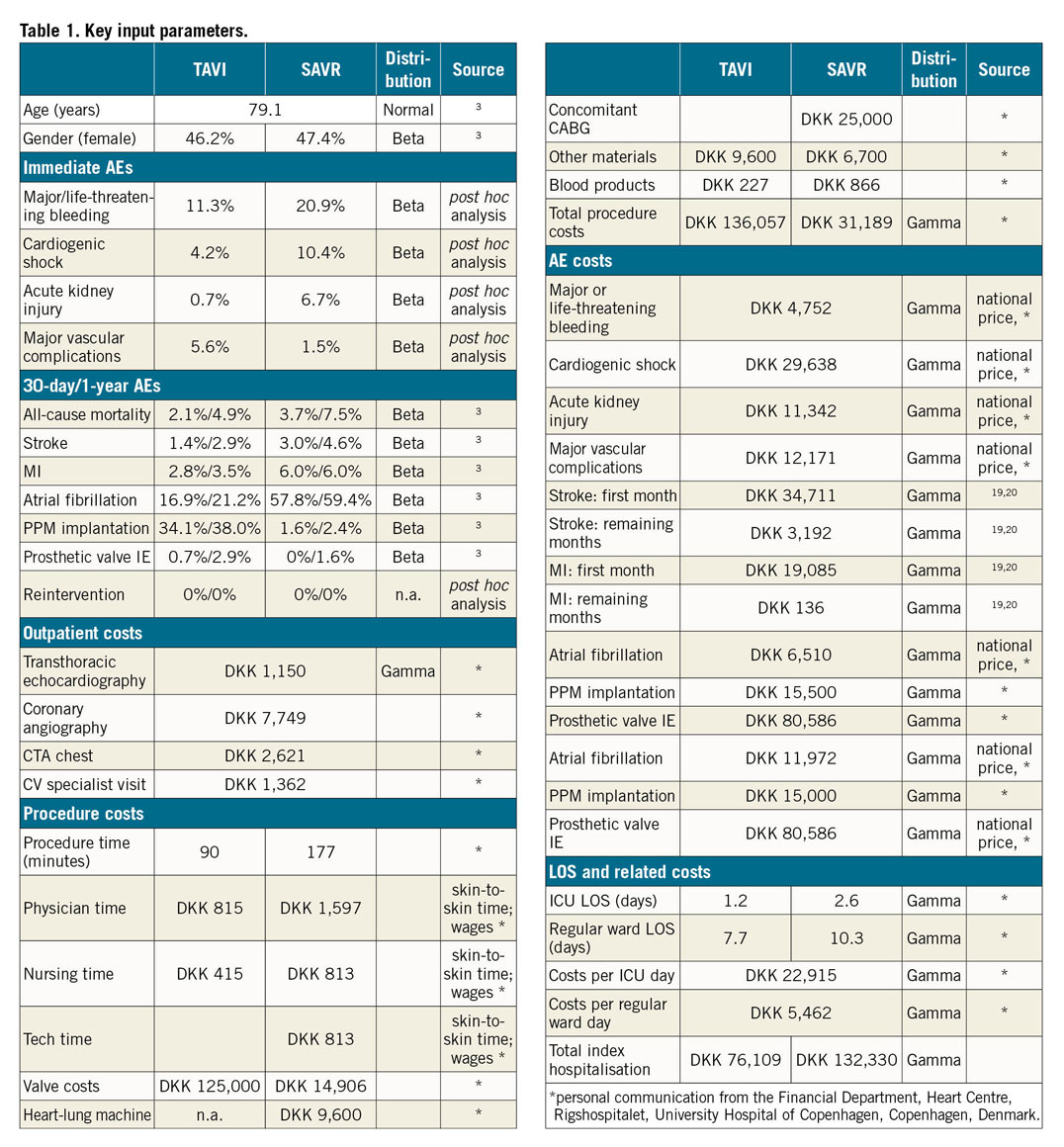
A Markov state transition model, nested in a decision tree (Figure 1), was developed to compute long-term costs and effectiveness of TAVI versus SAVR from the NOTION trial, as well as to quantify the impact of variation in the following input parameters: trial-based efficacy and adverse events, procedure and event costs, and New York Heart Association (NYHA) class-stratified health-related quality of life estimates scored for the Danish population. Patient-level data from the six-year follow-up were analysed wherever possible. The study adheres to both the American College of Cardiology/American Heart Association Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures as well as the Consolidated Health Economic Evaluation Reporting Standards statement5,6. The input parameters are shown in Table 1 and Supplementary Table 1-Supplementary Table 5. The complete methods are described in Supplementary Appendix 1-Supplementary Appendix 6.
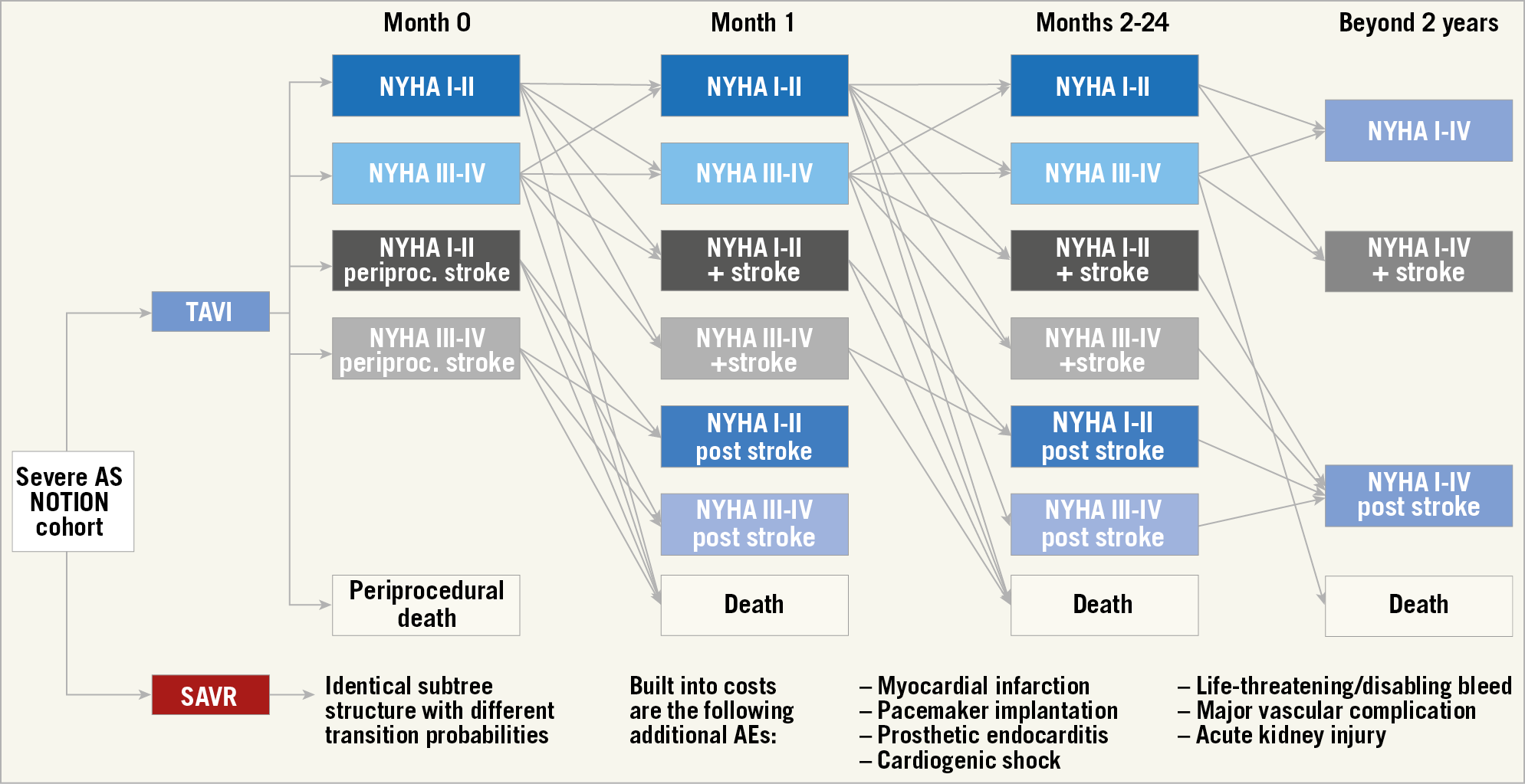
Figure 1. Model structure. Patients start with the index procedure and associated risk of periprocedural adverse events (AE): death, stroke, MI, major or life-threatening bleeding, cardiogenic shock, acute kidney injury, major vascular complication, atrial fibrillation, permanent pacemaker implantation, prosthetic valve endocarditis, or the need for reintervention. Mortality, stroke, and MI probabilities and utilities were calculated separately for the following states: NYHA Class I/II, NYHA Class III/IV, NYHA Class I/II after an MI, NYHA Class III/IV after an MI, NYHA Class I/II after a stroke, and NYHA Class III/IV after a stroke. All other AE consequences were included in transition probabilities and utilities for these strata, but costs occurred separately as per the calculated event probabilities.
Results
MODEL CALIBRATION AND BASE CASE FINDINGS
After calibrating the available 60 months of data, the model output matched the actual trial results for the endpoints all-cause mortality, stroke, and MI (Supplementary Figure 1-Supplementary Figure 3).
The model-projected life expectancy was 8.95 and 8.76 life years for TAVI and SAVR, respectively, a difference of 69 days. Consequently, TAVI and SAVR patients would have lived until 88.0 and 87.9 years of age, respectively.
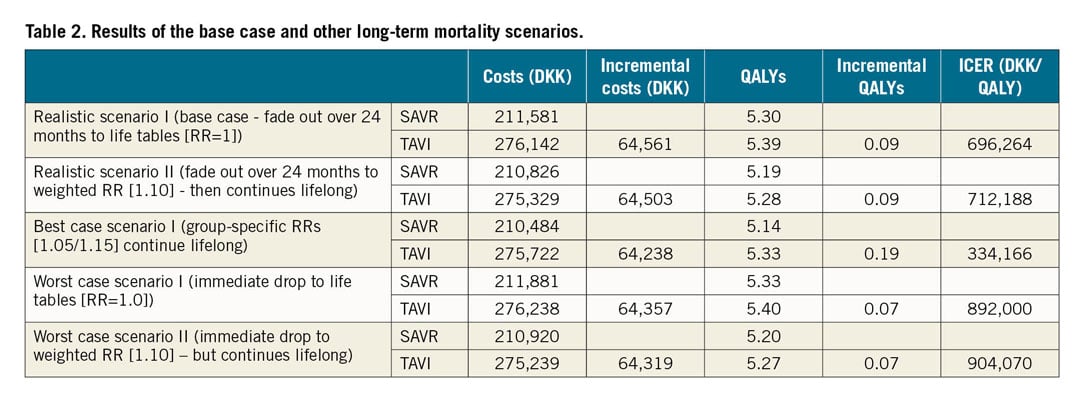
In the base case, TAVI was associated with total costs that were about DKK 65,000 higher than SAVR (276,142 vs 211,581), but TAVI patients also accumulated 0.09 additional quality-adjusted life years (QALYs; 5.39 vs 5.30). The resulting incremental cost-effectiveness ratio (ICER) of DKK 696,264/QALY (around €72,100/QALY using purchasing power party [PPP]-adjusted conversion via US dollars; 1 DKK=€0.1036) was below the willingness-to-pay (WTP) threshold for cost-effective (~DKK 1.1 million/QALY) and above the WTP threshold for highly cost-effective (DKK 375,489/QALY). The discounted ICER with quality-unadjusted, but still discounted, life years as effectiveness parameters was DKK 409,011/life year gained (Table 2, Supplementary Table 6).
UNCERTAINTY ANALYSES
Sensitivity analyses revealed that the model projections were robust in terms of limited sensitivity to variation of short-term complications, except for the following parameters: relative mortality risks for TAVI and SAVR over 60 months, periprocedural TAVI mortality, and relative risk of mortality post stroke. Figure 2 and Supplementary Figure 4 display the results of a set of key deterministic one-way sensitivity analyses in a tornado diagram. As per a threshold analysis, large differences in mortality risk (e.g., initial mortality RR of 0.71 for SAVR – and assumed concurrent RR for TAVI of 1.23) would push the results above the WTP threshold for cost-effectiveness. Likewise, if the short-term absolute TAVI mortality was more than 1.7% higher than the SAVR mortality or if the relative risk of stroke was under 0.72, TAVI would not be cost-effective. Variation of all other input parameters across defined ranges did not change the decision; the largest impact was seen when assessing different TAVI procedure and SAVR index hospitalisation – in particular the length of the intensive care unit (ICU) stay, as well as the length of stay on the regular ward for both procedures.
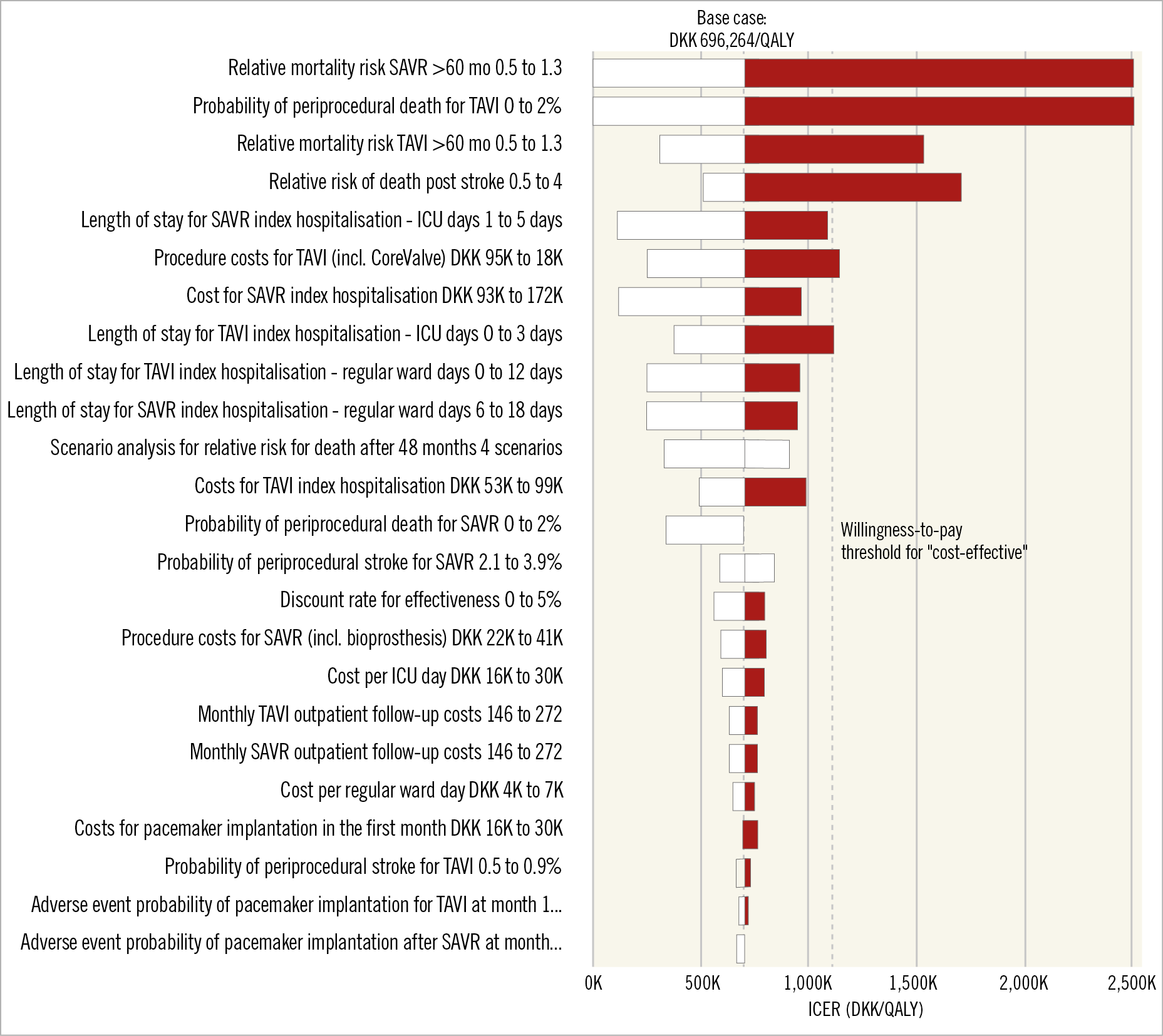
Figure 2. One-way sensitivity analyses in the form of a tornado diagram. Data categories are listed vertically instead of the standard horizontal presentation, and the categories are ordered so that the largest bar (based on the largest spread of the ICER) appears at the top of the chart, the second largest appears second from the top, and so on. A black bar represents the ICER for the high value of the varied input parameter and a white bar the low value. The base case and the willingness-to-pay threshold for “cost-effective” are marked with dashed lines.
Figure 3 depicts the results of the probabilistic sensitivity analysis. About 42% of the 5,000 simulations resulted in an ICER under DKK 375,489 whereas 78% of the simulations were below DKK 1,126,467 (Supplementary Figure 5-Supplementary Figure 7).
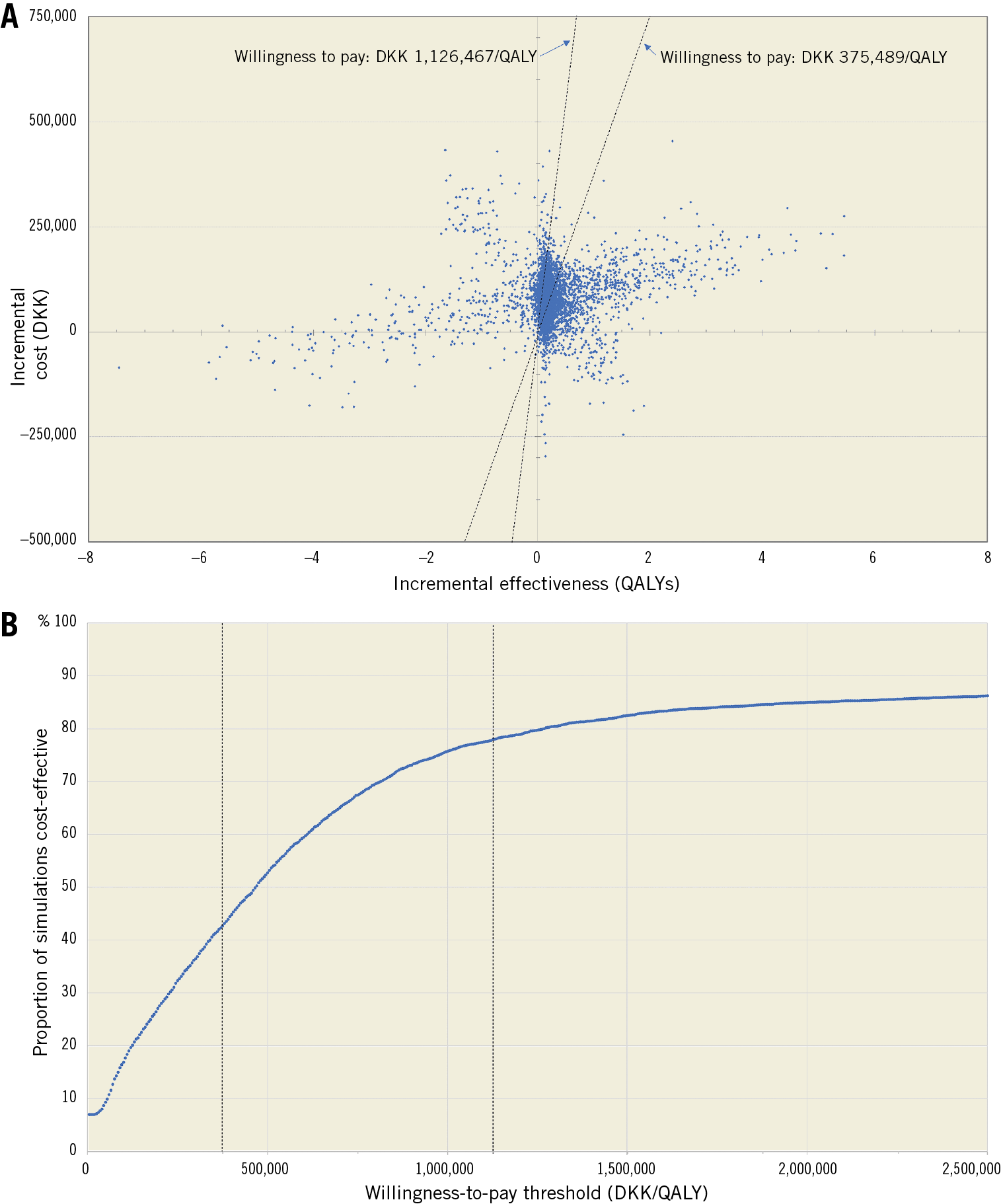
Figure 3. Probabilistic sensitivity analysis. Panel A shows a scatter plot containing the 5,000 simulation results as sets of incremental costs (y-axis) and incremental effectiveness in QALYs (x-axis). The two willingness-to-pay (WTP) thresholds of DKK 375,489 and DKK 1,126,467 are marked as dashed lines. Panel B contains the corresponding WTP threshold where the y-axis depicts the percentage of simulations that are cost-effective at the given WTP threshold on the x-axis.
Mortality was singled out as the most important driver for cost-effectiveness. Therefore, the RR for long-term mortality (i.e., beyond the observed 60-month period) was assessed further in sensitivity and scenario analyses (Table 2, Supplementary Figure 8-Supplementary Figure 16). As expected, the continued benefit scenario as the most optimistic scenario provided the lowest ICER (DKK 334,166/QALY), while the stop-and-drop scenarios as the most conservative scenarios provided the most pessimistic results (ICERs of DKK 892,000/QALY and DKK 904,070/QALY for the stop-and-drop scenario with an immediate drop to life tables and with a drop to the weighted relative risk, respectively). The fade-out scenarios, which were considered the most realistic, resulted in ICERs between those two extreme scenarios (DKK 696,264/QALY and DKK 712,188/QALY for fading out to life tables and to weighted relative risk, respectively). Importantly, the two-way sensitivity analyses (Supplementary Figure 10, Supplementary Figure 14) indicate that the width of the “corridor” where TAVI (compared to SAVR) is highly cost-effective and not just cost-effective or is dominated by SAVR depends on which scenario is analysed, as evidenced by the difference of the blue shapes in those figures: while the corridor for the realistic scenario is relatively broad, the best case scenario was more susceptible to changes to the long-term mortality.
Discussion
The present study is the first economic evaluation to compare first-line TAVI with SAVR in a cohort comprised primarily of patients at low surgical risk. Its results indicate that the additional resources spent on TAVI are, on average, well spent given that there were no statistical differences in the underlying clinical trial3 and the health economic profile is favourable. While TAVI is not an economically dominant or cost-saving procedure in this setting, all examined scenarios fell below the cost-effective WTP threshold set by the World Health Organization, and in one scenario TAVI was highly cost-effective.
The reason for this perhaps initially counterintuitive result – that TAVI is cost-effective – is that the higher TAVI device price is partially compensated for by the lower procedural and hospitalisation costs, as well as the slightly lower major adverse event rates. The only exception for this is permanent pacemaker (PPM) implantation due to a high risk of conduction abnormalities such as complete heart block among TAVI patients. The incidence of PPM placement was 34.1% at 30 days compared with 1.6% in patients treated with SAVR3, a value that was higher than rates found in contemporary trials or registries that have studied self-expanding TAVI devices7,8,9. Given the costs of only around DKK 15,500 (excluding hospital stay) for PPM implantation in the Danish setting (personal communication from the Financial Department, Heart Centre, Rigshospitalet, University Hospital of Copenhagen, Copenhagen, Denmark), this difference in PPM rates did not have a greater impact on the results. This might be different in other healthcare systems where PPM implantation is associated with higher costs. However, data from newer studies of self-expanding TAVI devices, including Evolut™ R (Medtronic, Minneapolis, MN, USA), suggest a reduction in observed PPM rates that can be expected to improve the cost-effectiveness of TAVI10.
On the other hand, the largest drivers of the value of TAVI in the NOTION trial were associated with periprocedural and long-term mortality risk, as well as the costs for the procedure itself and the index hospitalisation.
MORTALITY
Even though four-year results have recently been made available for the NOTION trial (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017), the impact of just changing the relative all-cause mortality risk of 0.05 to 0.1 (compared to the general population) beyond those four years resulted in significant changes in the incremental QALYs; while the worst case scenarios resulted in a difference of only 0.07 QALYs, the best case scenario showed a benefit of around 0.19 additional QALYs for TAVI patients. It was these incremental QALY differences that drove the somewhat different ICERs, and the base care that we chose, one of the fade-out scenarios (termed “realistic” scenario), was a middle ground between the possibly too conservative stop-and-drop scenarios and the possibly too optimistic continuous-benefit scenarios.
While we varied the RRs for the long-term mortality in a large range of parameters, the one-way analyses should be interpreted with caution. For example, the ICER of nearly DKK 2.8 million/QALY gained for a SAVR RR of 0.5 would only have an effect if the TAVI RR stayed at 1.05, which is unrealistic based on the four-year follow-up results from NOTION (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017). Therefore, the two-way sensitivity analyses can be helpful, providing reassurance that even when increasing or decreasing the RR for SAVR a bit more than the one for TAVI would still result in a similar ICER range; in particular, the two-way sensitivity analysis for the realistic scenario contained a broad cost-effectiveness “corridor”.
Most importantly, a difference in periprocedural mortality between TAVI and SAVR of 2% will render the procedure cost-ineffective. However, it is not likely that the periprocedural mortality of TAVI in other cohorts will exceed that of SAVR given that there was no statistically significant difference in the low-risk NOTION cohort3,4 (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017) and the intermediate-risk SURTAVI cohort8, and that it was superior at the initial follow-up of the CoreValve High Risk cohort7. The same holds true for data observed in previous trials of balloon-expandable TAVI devices11,12.
TAVI PROCEDURE COSTS
While comments about future device price developments would be speculative, it is not just possible but seems highly likely that, with increasing procedural experience, procedures and discharge processes will be streamlined, and other costs associated with TAVI will decrease further13,14.
HOSPITALISATION COSTS
The costs for the index hospitalisation are considerable - DKK 132,330 after SAVR, not including additional costs for various procedure-related adverse events. SAVR has been performed for decades, and discharge from both the ICU and regular floors can be considered to have little possible room for efficiency gains given the maturity of the procedure, even though performance might vary between surgeons and centres15. In particular, length of stay might be slightly longer in the Nordic countries compared to the USA and certain other European countries. What seems sensible to point out is that, after these long hospitalisations and procedure-related adverse events, old and frail patients will have a harder time recovering from “post-hospital syndrome”16 – so every day less spent in the hospital, and in particular in ICU settings, might be even more meaningful for the relatively old patients with severe aortic stenosis and their relatives than can be gathered in these data. On the other hand, fast-track hospital stay and even next-day discharge have been developed for selected patients after TAVI13, and the length of stay after TAVI in the NOTION trial might have improved since the patients for this study were recruited between 2009 and 2014.
SURGICAL RISK
Compared with cost-effectiveness analyses in high or extreme surgical risk cohorts2, the additional benefit associated with TAVI that was found in the present study is smaller. Given that the NOTION trial was a low surgical risk cohort, different results are to be expected. The relatively small effectiveness gain is in line with the clinical results from the low-3,4 (Søndergaard L. Clinical, safety and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) trial: Four-year follow-up data in all-comer patients with severe aortic valve stenosis. Presented at EuroPCR, Paris, France, 2017) and intermediate-risk8 cohorts and underscores that the additional expenditures for TAVI are partially compensated for by other cost-saving mechanisms, including the lower procedure costs apart from the device costs, the lower index hospitalisation costs, and somewhat lower complication costs apart from PPM.
Limitations
Our study is subject to several limitations. First, the present analyses apply only to Denmark as different cost structures in different healthcare systems might lead to different results. Nevertheless, if potentially different cost structures were applied in a U.S. setting, then this would translate to a PPP-adjusted ICER of around $102,000/QALY gained which, per the ACC/AHA guidelines on costs and value17, can be considered a cost-effective intervention of an intermediate value. Second, a decision-analytic model is just a representation of the reality and will never be perfect. However, modelling might help to inform decision makers better, and it highlights areas in which uncertainty exists and possibly even quantifies this uncertainty. Third, the long-term mortality of TAVI and SAVR beyond four years is unclear. However, we have conducted extensive sensitivity and scenario analyses. Fourth, it is unclear whether the outcomes of the NOTION trial are applicable to other settings, for example in patients with another distribution of access routes, surgical risks, and comorbidities. However, the NOTION trial was the first to study TAVI in a low surgical risk cohort, and insights from the Danish setting provided valuable insight for other trials and observational studies in either low or intermediate surgical risk cohorts. Fifth, cost structures and absolute costs, in particular for the procedure itself and for the index hospitalisation, tend to vary dramatically between the USA and other countries18; consequently, predictions about the cost-effectiveness of self-expanding TAVI devices in other settings may prove difficult. Sixth, while some cost estimates were derived from national prices on a near-cost basis, other costs and prices were taken from a single Danish institution. However, TAVI and SAVR are performed in only four Danish centres, and costs are likely to be relatively similar among them. Finally, the results herein might be applicable only to self-expanding and not to balloon-expandable TAVI devices.
Conclusions
TAVI was shown to be cost-effective in a low surgical risk cohort. However, the results are highly dependent on the long-term mortality and are applicable only to Denmark or to countries with similarly structured direct medical costs. While model-based analyses can help to facilitate an appreciation of the cost and effectiveness drivers, long-term registries should be pursued to decrease the uncertainty around long-term mortality. Likewise, cost-effectiveness analyses should be conducted in other countries to prove or disprove the cost-effectiveness of TAVI in other low-risk settings.
|
Impact on daily practice TAVI might not just be an option for all patients (regardless of their surgical risk) with severe aortic stenosis in that its results are clinically not different from SAVR, but could also be cost-effective, depending on the setting. The suitability of individual patients for a transcatheter procedure should ultimately be decided on and the actual procedure performed by a dedicated service as both outcomes and costs might depend on the experience and procedural skill of that service. While the cost-effectiveness of TAVI has been demonstrated for low-risk patients in Denmark, different cost structures or long-term mortality in other countries and settings, e.g., in patients at other surgical risks, in newer self-expanding or in balloon-expandable devices, might result in other conclusions regarding their health economic profiles. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
J.B. Pietzsch and B.P. Geisler (Wing Tech Inc.) provided health-economic consulting services to Medtronic plc. L. Søndergaard was the principal investigator for the NOTION trial and has received a consultant fee and institutional research grants from Medtronic plc. H.G.H. Thyregod was the other principal investigator for the NOTION trial and has no conflicts of interest to declare. The other author has no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

