Abstract
Aims: We compared epicardial and microvascular reperfusion, infarct size, and clinical outcomes after primary and rescue PCI with and without GuardWire distal protection in patients with LAD vs. non-LAD acute myocardial infarction (AMI). In the general AMI population undergoing primary PCI, the use of GuardWire did not yield higher reperfusion success, reduced infarct size, or enhanced event-free survival. Whether GuardWire is beneficial in patients with AMI in certain territories of the coronary circulation is unknown.
Methods and results: In the EMERALD trial, 501 patients with AMI were randomised to PCI with vs. without distal protection. The outcomes were analysed as a function of culprit vessel (LAD vs. non-LAD) and the use of GuardWire. Patients with LAD vs. non-LAD infarcts had significantly (P≤0.0001) lower rates of final TIMI flow grade 3 (85.2% vs. 94.4%), myocardial blush grade 3 (40.1% vs. 67.6%), and complete ST-segment resolution (35.1% vs. 79.6%). Patients with LAD infarcts also had larger infarct size (25.8±21.8% vs. 11.3_13.7%, p<0.0001) and a trend towards higher rates of 6-month mortality (5.5% vs. 2.1%, p=0.09) and new-onset severe heart failure (3.5% vs. 1.1%, p=0.08). Rates of reperfusion were not related to GuardWire use in patients with LAD infarcts. In patients with non-LAD infarcts, the use of GuardWire was associated with a trend towards better epicardial and microvascular reperfusion.
Conclusions: Myocardial infarction in the territory of the LAD is associated with worse epicardial and microvascular reperfusion and worse 6-month clinical outcomes. Use of the GuardWire showed a trend towards better epicardial and microvascular flow in patients with a non-LAD infarct-related artery.
Introduction
Restoration of normal epicardial flow in the infarct-related artery is crucial to improving the prognosis of patients undergoing primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI).1,2 However, complete restoration of epicardial flow by achieving Thrombolysis in Myocardial Infarction (TIMI) flow grade 3 following reperfusion therapy in AMI does not necessarily translate to flow restoration on the tissue level or to microvascular reperfusion. Evaluation of microvascular status using myocardial blush grade and electrocardiographic ST-segment resolution (STR) provides important prognostic information above and beyond epicardial flow.3-6 Distal embolisation of thrombotic material and plaque debris during primary PCI has been hypothesised as a plausible mechanism of microvascular impairment and provided a rationale for the use of embolic protection devices during PCI to prevent embolic particles from passing into the distal microcirculation and thereby potentially improving microvascular perfusion. Disappointingly, however, in randomised trials, use of embolic protection devices did not result in greater reperfusion success, improved microvascular flow, reduced infarct size, or enhanced event-free survival.7-12 Notwithstanding, two recent meta-analyses evidenced improved outcomes after PCI for AMI using currently available thrombus aspiration devices, but not embolic protection or mechanical thrombectomy devices.13,14
In the randomised Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial, AMI in the territory of the left anterior descending artery (LAD) compared to AMI in other major epicardial arteries was associated with worse epicardial and tissue-level perfusion, larger infarct size and less favourable clinical outcomes.15 Whether use of embolic protection devices is beneficial in patients with AMI in certain territories of the coronary circulation is unknown. We therefore compared epicardial flow, myocardial perfusion and infarct size after primary PCI with and without the GuardWire distal protection in patients presenting with LAD infarcts vs. non-LAD infarcts (in the territory of the left circumflex or right coronary artery) in the prospective, randomised, multicentre Enhanced Myocardial Efficacy and Removal by Aspiration of Liberated Debris (EMERALD) trial.
Methods
The EMERALD trial protocol has been previously described.7 Briefly, a total of 501 patients ≥18 years of age presenting with cardiac symptoms consistent with AMI between 30 minutes and six hours in duration and ST-segment elevation of ≥2 mm in ≥2 contiguous leads-or with new left bundle-branch block-in whom primary or rescue PCI was intended and with coronary anatomy suitable to receive PCI were randomised to PCI with or without the 0.028» GuardWire Plus™ balloon occlusion and aspiration system (Medtronic, Santa Rosa, CA, USA).
Exclusion criteria were major surgery or active bleeding within six weeks; allergy to aspirin, thienopyridine, or heparin; neutropenia; thrombocytopenia; hepatic dysfunction; renal insufficiency (serum creatinine level >2.5 mg/dL); cardiogenic shock; non-cardiac condition with expected survival <1 year, need for multivessel intervention during the index procedure, significant narrowing of the left main coronary artery and requirement for coronary artery bypass graft surgery within 30 days.
Primary endpoints were the rate of complete (>70%) STR measured by continuous digital 12-lead Holter monitoring (Northeast Monitoring 180+, Namic, MA, USA) 30 minutes after last contrast injection and infarct size (percentage involvement of the left ventricle) as assessed by technetium-99m-sestamibi imaging five to 14 days post-procedure.6 Secondary endpoints included final TIMI flow, restoration of normal myocardial blush grade (MBG), and the 1- and 6-month composite and component rates of major adverse cardiac events (MACE) related to left ventricular dysfunction (death, new-onset sustained hypotension, new-onset severe heart failure, and hospital readmission for left ventricular dysfunction); and the 1- and 6-month composite and component rates of MACE related to ischaemia (death, reinfarction, ischaemic target vessel revascularisation, and disabling stroke). TIMI flow and myocardial blush at baseline and post PCI were graded on a scale of 0 to 3 using methods previously described.2,5 Corrected TIMI frame counts (CTFC) were calculated based on the number of cine frames required for dye to first reach standardised distal coronary landmarks.16
Angiographic parameters, STR and infarct size were analysed by independent core laboratories blinded to treatment allocation. Both primary and secondary clinical endpoints were adjudicated by a clinical events committee that was also blinded to treatment allocation.
Statistical analysis
Categorical variables were compared using the Fisher exact test for pairwise comparison. Continuous variables were presented as median with interquartile ranges (IQR) or as mean ±1 SD, and were compared by the Kruskal-Wallis test.
Stepwise logistic regression analysis was employed to determine independent predictors of TIMI flow, CTFC, complete STR, MBG, infarct size, and 6-month mortality. Candidate variables for multivariable analysis included age, gender, diabetes, hypertension, hyperlipidaemia, smoking, body mass index, triple-vessel disease, prior myocardial infarction and/or bypass surgery, Killip class ≥2, time from symptom onset to first balloon inflation, maximal and minimal pre-procedure heart rate, maximal and minimal pre-procedure systolic and diastolic blood pressure, baseline haematocrit, use of platelet glycoprotein IIb/IIIa inhibitors, randomisation to GuardWire, LAD infarct-related artery, pre-intervention TIMI flow 0/1, presence of collaterals and reference vessel diameter.
Results
Baseline characteristics
Baseline demographic and angiographic characteristics of 186 patients with LAD infarcts and 289 patients with non-LAD infarcts from the EMERALD trial are shown in Table 1.
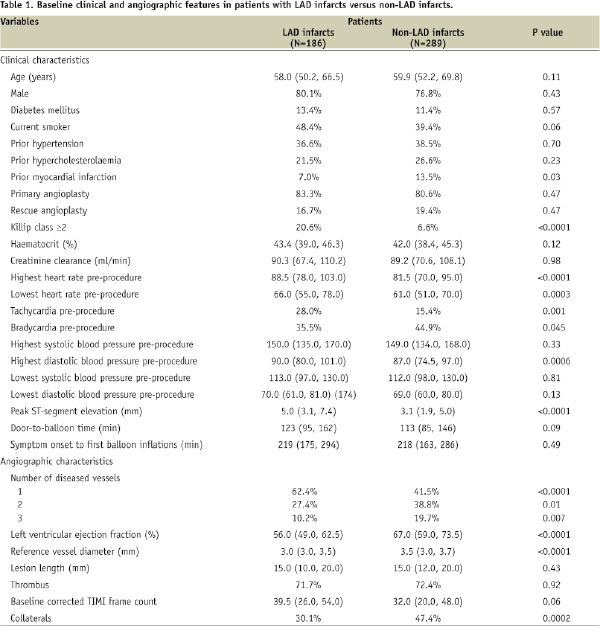
Patients with LAD infarcts compared to patients with non-LAD infarcts were less likely to have prior myocardial infarction, more likely to present with Killip class ≥2, had higher heart rate and diastolic blood pressure pre-procedure, higher degree of baseline ST-segment elevation, higher incidence of 1-vessel disease, smaller reference vessel diameter, lower left ventricular ejection fraction and a trend towards higher baseline CTFC. Collateral flow was observed less frequently in patients with LAD infarctions with higher incidence of Rentrop grade 1 (51.8% vs. 28.7%, p=0.003), lower incidence of grade 2 collaterals (26.8% vs. 49.3%, p=0.006) and a similar incidence of Rentrop grade 3 collaterals (21.4% vs. 22.1%, p=1.00). Time from symptom onset to first balloon inflation and door-to-balloon time did not differ significantly between the two groups. As shown in Figure 1A, the majority of patients in both groups had TIMI flow grade 0 or 1 on baseline angiography without significant differences between the groups.
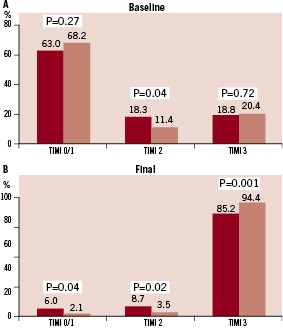
Figure 1. Baseline (A) and final (B) Thrombolysis in Myocardial Infarction (TIMI) flow in patients with LAD (dark bars) and non-LAD (light bars) myocardial infarction.
However, TIMI flow grade 2 pre PCI was observed significantly more frequently in patients with LAD infarcts. Rates of baseline MBG did not differ between the two groups (Figure 2A).
Figure 2. Baseline (A) and Final (B) Myocardial Blush Grade (MBG) in patients with LAD (dark bars) and non-LAD (light bars) myocardial infarction.
Procedural results
Procedural results and angiographic outcomes are shown in Table 2.
Rates of aspiration and balloon occlusion were similar in patients with LAD and non-LAD infarcts, though the median volume of aspirate was greater in patients with LAD infarcts. Peak creatine kinase was higher in patients with LAD infarcts. Angiographic complications were rare and occurred with similar frequency in both groups. Intra-procedural complications including hypotension, sustained ventricular tachycardia or ventricular fibrillation, conduction disturbances, or extreme bradycardia requiring treatment were more frequent in patients with non-LAD infarcts, whereas heart failure was more likely to develop in patients with LAD infarcts. Platelet glycoprotein IIb/IIIa inhibitors were less frequently administered to patients with LAD infarcts, and volume of contrast media was significantly higher in patients with LAD infarcts.
At the end of PCI, patients with LAD infarcts compared to patients with non-LAD infarcts had significantly lower rates of TIMI flow grade 3 and final MBG 3 with correspondingly higher rates of TIMI flow grades 0/1 and 2 and MBG 0/1 and 2 (Figures 1B and 2B). Median CTFC was significantly higher in patients with LAD vs. non-LAD infarcts (24.0 [18.0, 36.0] vs.18.0 [13.0, 25.0], p<0.0001).
In patients with LAD infarcts, using vs. not using distal protection resulted in similar rates of final TIMI flow grade 3 (83.5% vs. 87.2%, p=0.54) and CTFC (21.5 [16.0, 36.5] vs. 24.0 [19.0, 33.0], p=0.56) with a trend towards higher rates of MBG 3 (45.7% vs. 35.8%, p=0.12). In patients with non-LAD infarcts, the use of distal protection was associated with a trend towards higher rates of final TIMI flow grade 3 (97.2% vs. 91.8%, p=0.07) and final MBG 3 (71.4% vs. 64.0%, p=0.20) and significantly lower final CTFC (16.0 [12.0, 24.0] vs. 19.0 [14.0, 26.0], p=0.039).
ST-segment resolution
As shown in Figure 3, complete STR at 30 minutes after final contrast injection was more than 2-fold less common in patients with LAD infarcts compared to patients with non-LAD infarcts (35.1% vs. 79.6%, p<0.0001).
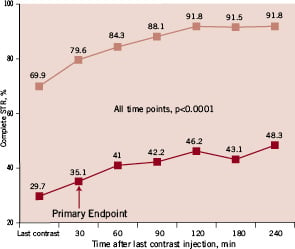
Figure 3. Rates of complete (>70%) ST-segment resolution (STR) at different time points in patients with LAD (dark squares) and non-LAD (light squares) myocardial infarction.
This difference continued to exist and remained highly significant (p<0.0001) at all other studied time points up to 240 minutes after final contrast injection. Correspondingly, patients with LAD vs. non-LAD infarcts were significantly more likely to have partial STR (30% to 70%) (43.1% vs. 16.8%, p<0.0001) and no STR (<30%) (21.8% vs. 3.6%, p<0.0001).
In patients with LAD infarcts, degree of complete STR was not related to the use of distal protection (31.5% vs. 38.8%, p=0.34), while in patients with non-LAD infarcts, use of distal protection was associated with a trend towards higher rates of complete STR (83.3% vs. 76.1%, p=0.14).
Infarct size
By Tc-99m sestamibi, patients with LAD infarcts compared to non-LAD infarcts had significantly larger mean (25.8_21.8% vs. 11.3_13.7%, p<0.0001) and median (22.5% [4.5%, 43.0%] vs. 8.0% [0.0%, 17.0%], p<0.0001) infarct size. Infarct size was not related to use of distal protection device either in patients with LAD infarcts (27.2±22.0% vs. 24.1±21.6%, p=0.36) or with non-LAD infarcts (11.6±13.2% vs. 10.9±14.1%, p=0.67).
Clinical outcomes
Thirty-day clinical outcomes were unrelated to LAD vs. non-LAD infarct vessel (Table 3).
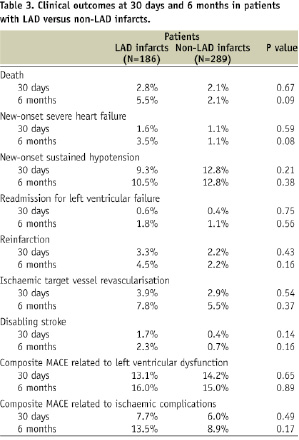
However, at six months, patients with LAD infarcts compared to patients with non-LAD infarcts had a trend towards increased mortality, higher incidence of new-onset severe heart failure, reinfarction, disabling stroke, and composite MACE related to ischaemic complications. Rates of new hypotension, ischaemic target vessel revascularisation, and MACE related to left ventricular dysfunction were nearly identical in both groups. Occurrence of clinical events was not related to the use of distal protection either in patients with LAD infarcts or with non-LAD infarcts.
Multivariable analyses
As shown in Tables 4 and 5, multivariate analysis demonstrated that LAD infarct vessel was a significant, independent predictor of failure to achieve TIMI flow grade 3 and MBG 3, as well as slower CTFC, incomplete STR, and larger infarct size.
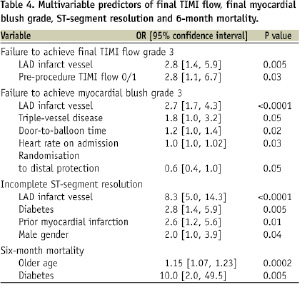
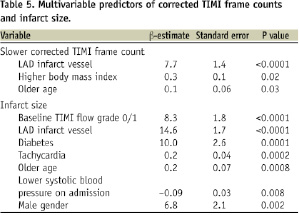
Randomisation to distal protection was the only independent predictor of achieving final MBG 3. Independent predictors of 6-month mortality included diabetes and older age, but not LAD infarct vessel (OR = 1.85, 95% CI 0.39 to 8.70, p=0.44).
Discussion
The principal findings of this study are: 1) Mechanical reperfusion in patients with AMI in the territory of the LAD compared to patients with non-LAD infarcts was associated with worse final epicardial flow and microvascular perfusion, resulting in larger infarct size and a trend towards higher rates of 6-month mortality and new-onset heart failure; 2) In patients with AMI in the territory of the LAD, use of the GuardWire distal protection device did not significantly alter epicardial flow, STR, infarct size, or clinical outcomes; 3) In patients with non-LAD infarcts, the use of a distal protection device was associated with a trend towards better epicardial and microvascular reperfusion and higher rates of complete STR, although this did not translate into smaller infarct size or improved clinical outcomes; 4) Infarct in the territory of the LAD was an independent predictor of failure to achieve TIMI flow grade 3, MBG 3, and complete STR, and a predictor of larger infarct size.
Consistent with previous data, patients in the EMERALD trial with LAD vs. non-LAD infarcts had significantly lower rates of successful anterograde flow and tissue-level reperfusion, resulting in larger infarct size, more than 2-fold higher rates of mortality and reinfarction and more than 3-fold higher rates of new-onset heart failure and disabling stroke.7,15,17
Pathophysiologic mechanisms underlying the poorer reperfusion that results in larger infarct size and worse prognosis in patients with anterior MI are not entirely understood. In this study, patients with LAD vs. non-LAD infarcts had smaller reference vessel diameters, as well as less developed collaterals and a higher volume of thrombotic material was retrieved from the infarct vessel. However, neither reference vessel diameter nor the degree of collateral flow or volume of thrombotic mass predicted outcomes in these patients on multivariable analysis. Another potential mechanism is the fact that distal protection devices may cause embolisation to side-branches that are typically more present in the LAD territory. This may attenuate the possible effect of thrombus aspiration.
Platelet GP IIb/IIIa inhibitors were less frequently administered to patients with LAD vs. non-LAD infarct vessels. There is evidence that both epicardial flow and tissue level reperfusion may be improved with GP IIb/IIIa blockade, possibly preventing formation and embolisation of platelet aggregates into the distal circulation.18-20 However, in this analysis, when introduced into the multivariable model, GP IIb/IIIa inhibition did not predict improved flow.
The left anterior descending artery commonly supplies a larger myocardial mass which may create more damaged myocardium at microvascular level. Vasospasm, endothelial injury, embolisation by thrombus and/or atheroma, plugging of the capillaries with neutrophils, myocyte oedema and endothelial blistering have all been hypothesised as plausible mechanisms of impaired epicardial flow and microvascular perfusion.21,22 There is also an opinion that distal microvascular constriction is promoted by vasoactive substances produced by activated platelets, thus impeding flow.22,23 It is possible that patients with LAD vs. non-LAD infarctions have more severe vasoconstriction resulting in more pronounced impairment of tissue perfusion. This hypothesis provided a rationale for the use of potent vasodilators to decrease the degree of vasoconstriction and to reduce infarct size. In A Randomised, Double-Blinded, Placebo-Controlled Multicentre Trial of Adenosine as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction II (AMISTAD-II), infarct size was reduced with high-dose (70-µg/kg/min) adenosine infusion, and there was a trend towards improved outcomes in patients with anterior infarction.24
Despite the rate of technical success rate using the GuardWire, the distal embolic protection device was not related to the target vessel, the present analysis showed a trend towards better epicardial and microvascular reperfusion using distal protection in patients with non-LAD infarcts. Specifically, the use of distal protection in this subgroup was associated with significantly lower CTFC, demonstrating quantitatively faster coronary flow and a trend towards higher rates of epicardial TIMI grade 3 flow and MBG 3, with more patients also achieving complete STR. In contrast, epicardial flow, CTFC, and rates of complete STR did not differ as a function of distal protection in patients with LAD infarcts, although there was a trend towards a higher rate of final MBG 3 in patients with LAD infarcts in whom distal protection was applied. Of interest, after adjusting for other variables, MBG 3 was 40% more likely to be achieved in patients randomised to distal protection. Prospective studies should further address the usefulness of protection devices in different coronary vessels.
Limitations
As a secondary analysis with multiple statistical comparisons, this study should be viewed as hypothesis-generating rather than definitive. Because the trial was relatively small, it was underpowered to exclude small differences between the two groups, and thus lacks statistical power to examine relationships between laboratory and clinical endpoints within subgroups. Clinical follow-up was limited to six months. If the duration of follow-up had extended, it is likely that differences in clinical outcomes in relation to the use of distal protection device would be more apparent.
Conclusion
This analysis demonstrates that myocardial infarction in the territory of the LAD is associated with worse epicardial flow and microvascular reperfusion and a trend towards worse 6-month clinical outcomes. Use of the GuardWire distal protection device was associated with a trend for better epicardial and microvascular flow in patients with non-LAD infarct-related arteries. Future trials of devices aimed at protecting distal vessel circulation should be powered to assess outcomes in different coronary vessels.
Acknowledgements
The authors wish to express their gratitude to Jason Kahn for his unsurpassed assistance in the preparation of this manuscript.

