Abstract
Aims: Slow flow and no flow phenomena have been associated with distal embolisation, especially of plaque debris, and with unfavourable clinical outcomes. However, patients at high risk of distal embolisation for whom distal protection might be beneficial have not been adequately identified. We examined the frequency of distal embolisation and its predicting factors, including both ACS and non-ACS patients.
Methods and results: Consecutive patients (n=98) with or without ACS who had received PCI with a filter-type distal protection device and successful angioscopic and VH-IVUS examination were prospectively enrolled. The presence of yellow plaque and plaque rupture was evaluated by angioscopy. Tissue classification and plaque burden was evaluated by VH-IVUS. Distal embolisation was evaluated by pathological examination of material collected in the filter. Distal embolisation of plaque debris was more frequently detected in patients with ACS (48% vs. 25%, p=0.02), in those with ruptured plaque (86% vs. 13%, p<0.001), in those with large (>75%) plaque burden (50% vs. 23%, p=0.006), and in those with grade 2/3 yellow plaque (52% vs. 7%, p<0.001), as compared to those without it.
Conclusions: The presence of ruptured yellow plaque and of large plaque burden, rather than the setting of ACS, was highly predictive of distal embolisation of plaque debris.
Introduction
We sometimes experience slow flow or no flow phenomena during PCI of ACS and non-ACS patients, which makes the result of PCI considerably worse. High mortality rates have been reported in those patients with slow flow/no flow phenomenon1. Although distal protection devices have been developed to prevent this phenomenon and have demonstrated beneficial effects in the PCI of SVG lesions, the EMERALD trial could not demonstrate the beneficial effect in acute MI patients2. However, we have previously reported the beneficial effect of the device in a selected high-risk group of acute MI patients with ruptured plaque at the target lesion3. Distal embolisation of plaque debris was frequently demonstrated in those patients. On the other hand, the effect of the device for stable angina patients has not been established. Therefore, in the present study, using a filter-type distal protection device, we examined the frequency of distal embolisation and its predicting factors both in ACS and non-ACS patients.
Methods
STUDY DESIGN
This is a single-centre prospective study to clarify: 1) the frequency of distal embolisation and the potential usefulness of a filter-type distal protection device; and 2) the predictive parameters for distal embolisation using angioscopy and VH-IVUS. All ACS or non-ACS patients undergoing stent implantation at de novo lesions of native coronary arteries and evaluation of the target lesion by angioscopy and VH-IVUS were considered for this study from February 2008 to February 2010. Exclusion criteria were distal lesion location, vessel diameter <2.0 mm by online quantitative coronary angiography, and severe vessel tortuosity, which were all considered unsuitable for the use of a distal protection device.
Catheterisation was performed by femoral, brachial, or radial artery approach using a 6 Fr or 7 Fr sheath and catheters. Coronary angiogram was recorded using the Innova cardiovascular imaging system (GE Healthcare Japan, Tokyo, Japan), and quantitative coronary angiographic analysis was performed with the system to measure the percent diameter stenosis of the PCI target lesions. The target lesions of PCI were examined by angioscopy and VH-IVUS before PCI. Thrombus aspiration was performed in all ACS patients before PCI. Because the majority of culprit lesions of both acute MI and unstable angina are known to have disrupted yellow plaque and thrombus, we treated culprit lesions of both MI and unstable angina similarly by aspiration therapy first. Distal protection was performed in all enrolled patients with a filter-type distal protection device, Filtrap (Japan Lifeline Co., Ltd, Tokyo, Japan), during the PCI procedures.
IVUS was routinely used as a guide for all PCI procedures in our hospital. Although performing an angioscopic examination was encouraged, it was performed only when: 1) the target vessel was suitable for angioscopic examination (non-distal lesion location, vessel diameter >2 mm, and no severe vessel tortuosity); 2) the device and the angioscopy specialist were available at the time of catheterisation; and 3) adequate time was available for angioscopic examination. The distal protection device was introduced after IVUS and before angioscopic examination. Although the presence or absence of distal embolisation due to the imaging by IVUS or angioscopy was not demonstrated histologically, no case in the present study suffered slow/no flow immediately after imaging.
Filtrap is a single-use, steerable 0.014” fixed wire, equipped with a filter basket. During PCI procedures, plaque debris and thrombus are collected by the filter basket, whereas blood can flow freely through the porous membrane of the filter. At the end of the procedure, the filter retaining the embolic material is removed from the target vessel and processed for pathological examinations. Device success was defined as successful deployment and retrieval of the Filtrap without angiographic complications such as dissection and vasospasm. Filter slow/no flow was defined as the apparent reduction of coronary flow speed observed on the angiogram, i.e., TIMI flow grade <3, while a filter-type distal protection device was placed in the coronary artery. Angiographic slow/no flow was also evaluated at the end of the PCI procedure, after the removal of the distal protection device.
All enrolled non-ACS patients were taking aspirin 100 mg/day and ticlopidine 500 mg/day or clopidogrel 75 mg/day (dual antiplatelet therapy), and ACS patients who were not receiving dual antiplatelet therapy received a loading dose of aspirin 200 mg and clopidogrel 300 mg as soon as possible before PCI. Unfractionated heparin was administered to maintain an activated clotting time >250 seconds during the PCI procedures. GP IIb/IIIa inhibitors were not used for anyone, because they were not approved in Japan.
ACS included acute MI with/without ST-elevation as defined by The Joint European Society of Cardiology/American College of Cardiology Committee, and unstable angina was defined according to the Braunwald classification. Hypertensive patients were defined as patients with blood pressure >140/90 mmHg or those already taking antihypertensive drugs. Diabetic patients were defined as patients with fasting blood glucose >126 mg/dl or those already taking oral drugs for diabetes mellitus or receiving insulin therapy. This study was approved by the Osaka Police Hospital Ethical Committee. Written informed consent was obtained from all the patients enrolled.
ANGIOSCOPIC EXAMINATION AND EVALUATION
The angioscope RX-3310A and MV-5010A (Machida, Tokyo, Japan) and optic fibre DAG-2218LN (Machida, Tokyo, Japan) were used. The angioscopic observation of PCI target lesions was done while blood was cleared away from view by the injection of 3% dextran-40 as we have previously reported4. The yellow colour of detected plaque was classified into three grades: 1) slight yellow; 2) yellow; and 3) intense yellow, as compared with standard colours as we have previously reported4,5. Thrombus was defined as white or red material that had a cotton-like or ragged appearance or that presented fragmentation with or without protrusion into the lumen or adherent to the luminal surface. According to our previous report3, ruptured plaque was angioscopically defined as fulfilling two or more of the following criteria: 1) >50% of the luminal area occupied by thrombus or protruded plaque content; 2) presence of a large cavity or fissure; 3) yellow thrombus over yellow plaque; and 4) distal embolism observed by angioscopy. For each target lesion, the presence of yellow plaque (with its yellow colour grade) and the presence of ruptured plaque were determined. Two angioscopy specialists evaluated the angioscopic images blinded to patient characteristics. In case of disagreement, a third reviewer served as an arbitrator. The inter- and intra-observer reproducibility for the interpretation of angioscopic images was good for plaque colour (kappa=0.77 and 0.92), thrombus (kappa=0.79 and 1.00), and plaque rupture (kappa=0.73 and 0.85).
VH-IVUS EXAMINATION AND EVALUATION
VH-IVUS imaging was performed with a phased-array, 20-MHz, 3.2 Fr IVUS catheter (Eagle Eye; Volcano Corporation, Rancho Cordova, CA, USA) after intracoronary administration of 0.2 mg nitroglycerine. During pullback, greyscale IVUS was recorded and raw radio-frequency data were captured at the top of the R-wave. Reconstruction of the colour-coded map by a VH-IVUS data recorder was performed (S5; Volcano Corporation, Rancho Cordova, CA, USA). On each cross-sectional image of VH-IVUS, the area of each tissue classification (necrotic core [NC], fibro-fatty [FF], fibrous [F], and calcification [C]) was measured as a percentage of plaque area. Plaque burden was defined as plaque area/vessel area. The data from three cross-sectional images of the target lesion were averaged for the lesion. We defined NC-rich plaque as NC area >16% (median), FF-rich plaque as FF area >12% (median), F-rich plaque as F area >63% (median), C-rich plaque as C area >4% (median), and large plaque burden as plaque burden >75%.
PATHOLOGIC EXAMINATION AND EVALUATION
The tip of the Filtrap device (filter) was cut and processed for pathologic examination. The collected material was gently removed from the filter and processed for histological examination. When aspiration therapy was performed, aspirated blood was filtered and was processed for histological examination only when aspirated material was macroscopically visible on the filter. They were stained with haematoxylin and eosin, Masson trichrome stain, and Elastica van Gieson staining. Two expert pathologists blinded to patient characteristics analysed the specimens independently and, in case of disagreement, they discussed until they reached a consensus. The specimens were analysed for the presence of thrombus and for the presence of plaque debris (Figure 1). Thrombus was detected by the presence of thrombus material (fibrin, platelet aggregates, and red and white cells). Plaque debris was detected by the presence of atherosclerotic plaque tissue (extracellular matrix, necrotic gruel, fibrous fragments, foam cells, cholesterin crystals, and calcium deposits). Distal embolisation in the present study was defined as the presence of captured material (thrombus or plaque debris) in the filter device.
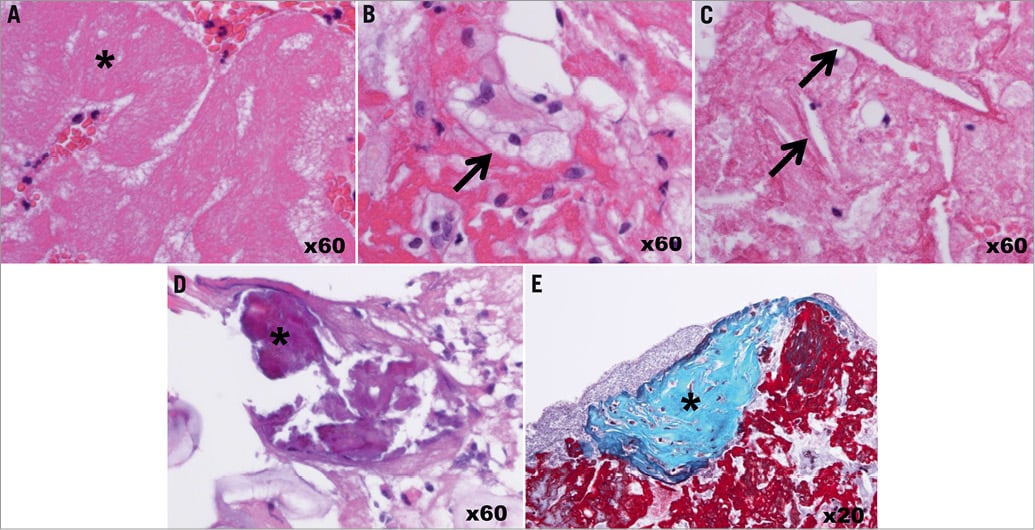
Figure 1. Pathological evaluations of thrombus and plaque debris. The collected material in the filter device was analysed for the presence of thrombus and for the presence of plaque debris. The presence of fibrin and platelet aggregates (* in A) indicated thrombus. The presence of foam cells (arrow in B), cholesterin crystals (arrow in C), calcium deposit (* in D), or fibrous tissue (* in E) indicated plaque debris. A, B, C, and D were stained with haematoxylin and eosin. E was stained with Masson trichrome.
STATISTICAL ANALYSIS
Continuous data were presented as mean±SD. Comparisons between groups were done by unpaired Student’s t-test or chi-square test. Multivariate stepwise logistic regression analysis was performed to examine the predictive factors for the distal embolisation of plaque debris or thrombus, including the following factors: hypertension, hypercholesterolaemia, diabetes mellitus, current smoking, angioscopic findings (yellow colour grade and plaque rupture), and VH-IVUS findings (percent necrotic core area, percent fibrofatty area, and plaque burden). A p-value <0.05 was regarded as statistically significant. Analysis was performed with SPSS version 16.0J for Windows (SPSS Inc., Chicago, IL, USA).
Results
STUDY PATIENTS AND PROCEDURAL RESULTS
Positioning of the Filtrap was attempted for 58 lesions in 58 ACS patients, and for 40 lesions in 40 non-ACS patients. Device success was attained in all 98 lesions. Evaluation of target lesions by angioscopy and VH-IVUS was performed in all of those patients. In 49 (50%) procedures (32 [55%] in ACS and 17 [43%] in non-ACS lesions), coronary flow decreased after stent implantation by the occlusion or sub-occlusion of the filter with the embolised material, i.e., filter slow/no flow, but returned to normal coronary flow after the removal of the distal protection device. Patients’, lesion and procedural characteristics are shown in Table 1.
ANGIOSCOPIC, VH-IVUS, AND PATHOLOGIC FINDINGS
Angioscopic and VH-IVUS findings are presented in Table 2 and pathologic findings in Table 3. Ruptured plaques were all yellow and were grade 1 in 0% (0/35), grade 2 in 46% (16/35), and grade 3 in 54% (19/35). Plaque rupture was detected in 47% (27/58) of plaques with large plaque burden. When plaques with vs. plaques without rupture were compared, mean plaque burden was not significantly different (76% vs. 74%) but the minimum value of plaque burden appeared higher in the ruptured plaques (63% vs. 46%). The prevalence of ruptured plaque was higher in ACS than in non-ACS patients (52% vs. 13%, p<0.001).
Plaque debris was captured by the distal protection device more frequently in ACS patients, patients with ruptured plaque, and patients with grade 2/3 yellow plaque compared with those without it. Thrombus was captured more frequently in patients with ruptured plaque, patients with grade 2/3 yellow plaque, and patients with large plaque burden compared with those without it. Among ACS patients, plaque debris was captured in 61% of acute MI patients and in 30% of unstable angina patients. Thrombus was captured in 89% of acute MI patients and in 65% of unstable angina patients. Remarkably, plaque debris was captured in 86% of patients with ruptured plaque. On the other hand, plaque debris was captured only in 7% of patients without grade 2/3 yellow plaque. Incidence of plaque debris embolisation for each type of target lesion is summarised in Figure 2.
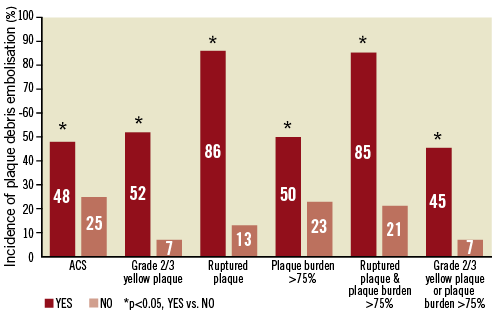
Figure 2. Frequency of plaque debris embolisation for each type of target lesion. Plaque debris embolisation was detected only in 48% of patients with ACS. However, it was detected in 86% of target lesions with ruptured plaque. Distal protection may be beneficial for these lesions. On the other hand, it was detected only in 7% of target lesions without grade 2/3 yellow plaque. We may be able to perform PCI relatively safely without distal protection for these lesions.
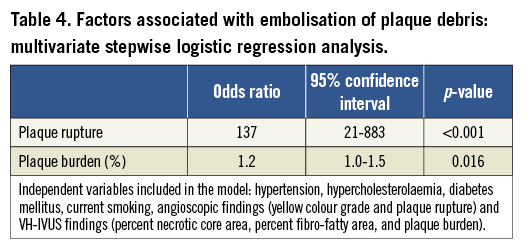
Multivariate analysis revealed ruptured plaque and plaque burden as independent factors significantly associated with embolisation of plaque debris when hypertension, hypercholesterolaemia, diabetes mellitus, current smoking, angioscopic findings (yellow colour grade and plaque rupture), and VH-IVUS findings (percent necrotic core area, percent fibro-fatty area, and plaque burden) were included in the analysis (Table 4); while current smoking (odds ratio 3.6, 95% confidence interval 1.1-12.4, p=0.03) was the only factor significantly associated with embolisation of thrombus.
Sensitivity, specificity, positive predictive value, and negative predictive value to predict plaque debris embolisation were 79%, 92%, 86%, and 87% for angioscopy (by the presence of ruptured plaque) and 76%, 52%, 50%, and 78% for IVUS (by plaque burden >75%), respectively.
Visually (macroscopically) detectable material was aspirated in 24% (14/58) of ACS patients. Thrombus and plaque debris were histologically confirmed in 86% (12/14) and 64% (9/14) of them, respectively. Distal embolisation of thrombus and plaque debris as captured by the distal protection device were detected in 93% (13/14) and 64% (9/14) of ACS patients with macroscopically detectable aspirated materials, respectively, while they were detected in 77% (34/44) and 43% (19/44) of patients without macroscopically detectable aspirated materials, respectively. Among 14 patients who had histological examination of aspirated materials, plaque debris embolisation was detected in 89% (8/9) and 20% (1/5) of patients with and without the retrieval of plaque debris, respectively. Although aspiration of thrombus and embolisation of thrombus had no significant correlation (R=–0.11, p=0.70), aspiration of plaque debris and embolisation of plaque debris had a significant positive correlation (R=0.69, p=0.006).
Plaque area evaluated by IVUS was reduced 2.0±2.5 mm2 by PCI. The reduction of plaque area appeared larger in the patients with versus those without plaque debris embolisation (2.7±2.4 mm2 vs. 1.6±2.5 mm2, p=0.05), and the incidence of plaque debris embolisation (52% vs. 29%, p=0.02) was significantly higher in the patients with versus those without plaque area reduction >1.6 mm2 (median).
Discussion
According to the results of the present study, distal embolisation of plaque debris was significantly associated with the presence of ruptured plaque and with large plaque burden, while the ACS setting was not selected as an independent risk factor. Remarkably, embolisation of plaque debris was detected in 86% of patients with ruptured plaque, and 13% of non-ACS patients had ruptured plaque. This may well be the first report which has demonstrated that the presence of ruptured plaque and large plaque burden are risk factors of distal embolisation in non-ACS patients.
IMPORTANCE OF PREVENTING DISTAL EMBOLISATION
The causes of no flow phenomenon observed in patients with acute MI are considered to be multifactorial: they have been classified as ischaemic injury, reperfusion injury, and distal embolisation. However, we sometimes experience the occurrence of slow or no flow that was not present before balloon inflation immediately after short-time balloon inflation not only in patients with ACS but also in patients with stable angina. Because the effect of ischaemia or reperfusion injury would be limited in that case, distal embolisation of thrombus and/or plaque debris during balloon inflation would be the main cause of slow flow/no flow phenomenon. Okamura et al displayed thrombus or plaque debris distal embolisation as high-intensity transient signals (HITS) using a Doppler guidewire in all patients with acute MI, which was completely prevented by distal protection6. Kotani et al demonstrated that a larger amount of plaque debris was aspirated from the target vessel when no flow occurred in patients with ACS7. Although GP IIb/IIIa inhibitors have been shown to reduce the incidence of periprocedural MI, we cannot expect as much from pharmacological therapy when large amounts of plaque debris are embolised. Myocardial contrast echo sometimes demonstrates no flow even when good reperfusion is observed by angiography8,9. A high mortality rate has been reported not only in patients with angiographic slow flow/no flow but also in patients with poor microvascular flow after PCI10,11. Although the EMERALD trial could not demonstrate the beneficial effect of the distal protection device2, we reported the reduction in the incidence of slow flow/no flow phenomenon and the improvement of left ventricular function by the occlusion-type distal protection device in acute MI patients with angioscopically detected ruptured plaque, in whom distal embolisation of plaque debris was frequently revealed3.
Although an occlusion-type distal protection device was effective to prevent slow flow/no flow phenomenon, it was difficult and complicated to use and might have had a relatively high rate of protection failure. The filter-type distal protection device is easier to use than the occlusion-type device and may be able to protect more efficiently12.
FACTORS ASSOCIATED WITH EMBOLISATION
As we have previously reported3, slow flow/no flow phenomenon occurs more frequently in patients with ruptured plaque at the target lesion of PCI than in patients without it, and distal embolisation of plaque debris is detected more frequently in those patients. Therefore, the embolisation of plaque debris may be an important cause of slow flow/no flow phenomenon, which is associated with the deterioration of left ventricular function and the worsening of clinical outcome. The predicting factors for the embolisation of plaque debris were examined in the present study and were identified as the presence of ruptured plaque and large plaque burden. Because no factors have ever been associated with distal embolisation in previous studies except for the setting/diagnosis of the patients, we performed multivariate analysis with various combinations of factors among the available factors in the present study. Medications were not significantly associated with distal embolisation. Patients had a very high (86%) risk of distal embolisation of plaque debris if they had ruptured plaque at the target lesion of PCI, regardless of whether the setting was ACS or non-ACS. Although the prevalence of ruptured plaque was higher in ACS than in non-ACS patients (52% vs. 13%, p<0.001) as was expected, it was still only half of ACS patients, and therefore ACS was not a strong predictor of ruptured plaque. On the other hand, the incidence of embolisation of plaque debris in patients without grade 2/3 yellow plaque at the target lesion was as low as 7%.
There have been some previous reports on the frequencies of thrombus or plaque debris embolisation2,3,6,13. The embolisation of thrombus was pathologically detected with a distal protection device in 95% of acute MI patients3, in 79% of unstable angina patients13, and in 70% of stable angina patients13. The embolisation of plaque debris was pathologically detected with a distal protection device in 69% of acute MI patients3, in 73% of ST-elevation MI patients2, in 21% of unstable angina patients13, and in 30% of stable angina patients13. The embolisation of thrombus or plaque debris was detected in 100% of acute MI patients by high-intensity transient signals (HITS) using a Doppler guidewire6. These previously reported results were consistent with the results of the present study. Therefore, we can generally say that the embolisation of plaque debris is detected in 60-70% of acute MI patients and in 20-30% of stable or unstable angina patients and, in the present study, its strongest predictor has been demonstrated to be the presence of ruptured plaque at the target lesion of PCI. Although the frequency of thrombus captured by distal protection device was consistently high, it varied from 48% (in cases without grade 2/3 yellow plaque) to 100% (in cases with ruptured plaque) depending on the condition of target lesions.
Theoretically, the plaques with a large necrotic core or large fibro-fatty tissue may have a high risk of plaque debris embolisation. However, NC-rich plaques or FF-rich plaques were not significantly associated with the embolisation of plaque debris in the present study. Furthermore, the area of NC or FF was not different between ACS and non-ACS patients. A plausible explanation for these results is that thrombus or a mixture of thrombus and protruded necrotic core is often classified as F by VH-IVUS; therefore, the area of F could have been overestimated and the area of NC could have been underestimated at the site of plaque rupture, which might have reduced the difference between ACS and non-ACS patients. Consequently, tissue classification by VH-IVUS may not be valid for lesions with thrombus and may not be useful to predict thrombus or plaque debris embolisation.
Aspiration therapy and examination of aspirated material may be useful to predict the occurrence of distal embolisation. However, we could not exclude the risk of plaque debris embolisation by the absence of macroscopically detectable aspirated materials, as plaque debris embolisation was detected in 43% of the patients who did not have macroscopically detectable aspirated materials. Prediction of plaque debris embolisation by the examination of aspirated material might be improved if all aspirated materials were histologically examined.
As the size of reduced plaque area by PCI was previously associated with the size of myocardial necrosis after PCI evaluated by magnetic resonance imaging14, it was also associated with the presence versus absence of plaque debris embolisation in the present study.
CLINICAL IMPLICATIONS
Although distal protection should be effective to prevent distal embolisation, it increases the cost and complexity of PCI procedures. We need to identify the patients or lesions with high risk of distal embolisation. According to the findings of the present study, patients with ruptured yellow plaque at the target lesion of PCI should benefit from the use of a distal protection device. Although ACS patients frequently have this kind of lesion and may benefit from the use of a distal protection device, non-ACS patients with this kind of lesion may also benefit from the use of a distal protection device. On the other hand, the incidence of plaque debris embolisation was 7% in patients without grade 2/3 yellow plaque and consequently the benefit of using a distal protection device may be very limited in these patients.
Ruptured plaques may be detected by optical coherence tomography (OCT) as well as by angioscopy. However, whether or not OCT can detect yellow plaques as well as angioscopy is unclear. Spectroscopy15 could be another promising new device to detect yellow plaque. While angioscopy gives us the information about macroscopic pathology in living patients, comparison studies between angioscopy and OCT or spectroscopy are needed to translate the findings by angioscopy into clinical practice where angioscopy is not available.
Study limitations
The important and valuable information of macroscopic pathology can be acquired by angioscopy in living patients, although angioscopy is not routinely used in daily practice. Distal embolisation may occur by crossing a Filtrap catheter through the culprit lesions. Some thrombus and debris may pass through the pores of the filter or sneak through the space between filter and vessel wall. Some of the embolised material may enter the side branches proximal to the filter. Some of the thrombus detected in the filter device may have been the result of in situ thrombus formation rather than the embolised thrombus, which might have resulted in the overestimation of the frequency of thrombus embolisation.
Each imaging modality may have had limitations in its sensitivity, specificity, or accuracy. According to previous validation studies using histology as the gold standard16,17, the specificity, accuracy, and predictive value of angioscopy to detect disrupted plaque were high (>90%) while its sensitivity was moderate (73%) due to its failure to detect small disruptions. Furthermore, the specificity, accuracy, and predictive value of angioscopy to detect thrombus were high (>93%) and its sensitivity was excellent (100%). Therefore, the angioscopic detection of ruptured plaque that has thrombus and large disruption would be reliable. On the other hand, VH-IVUS does not have criteria for detecting thrombus and often classifies thrombus as F, which might have influenced the results of the present study, although the predictive accuracy of VH-IVUS to detect F, FF, NC, and C has been reported as high (87-97%). Indeed, in the present study, the area adjacent to the lumen, where angioscopy revealed ruptured yellow plaque with thrombus, was often classified as F; therefore, the area of F should be overestimated and the area of NC underestimated.
We have not demonstrated the effectiveness of the device to prevent slow flow/no flow phenomenon, which remains to be shown in future randomised controlled clinical trials.
Conclusions
The presence of ruptured plaque and larger plaque burden at the target lesion of PCI were significantly associated with the higher incidence of plaque debris embolisation; in particular, 86% of patients with ruptured plaque had plaque debris embolisation. The filter-type distal protection device would be potentially beneficial for these high-risk patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

