- slow flow phenomenon
- plaque characteristics
- percutaneous coronary intervention
Abstract
Aims: To evaluate the plaque characteristics that predispose to the slow flow phenomenon during percutaneous coronary intervention (PCI). The slow flow phenomenon is a serious complication of PCI and is associated with poor prognosis. It is difficult to predict this phenomenon from greyscale intravascular ultrasound (IVUS) data obtained before PCI. iMap™ is a new software package for assessing plaque composition from data obtained by 40 MHz IVUS imaging.
Methods and results: Ninety-five consecutive patients underwent 40 MHz IVUS, including 33 with acute coronary syndrome. Plaque volume was calculated by IVUS and plaque components were detected by iMap software. Plaques were characterised as fibrotic, lipidic, necrotic, or calcified. Correlations among plaque characteristics and the slow flow phenomenon were analysed. Slow flow during PCI was observed in 11 patients (11.6%). Both the absolute volume and percentage of necrotic plaque were significantly higher in the slow flow group than the normal flow group (43.3±33.5 mm3 vs. 20.1±17.2 mm3, p=0.0004, 19.7±5.1% vs. 14.6±8.3%, p=0.047). Receiver-operating characteristic analysis showed that the necrotic plaque volume and necrotic plaque ratio were significantly better predictors of slow flow during PCI compared with total plaque volume. The cut-off value of necrotic plaque volume for predicting slow flow was 21.6 mm3 (sensitivity of 81.8% and specificity of 61.9%).
Conclusions: Characterisation of plaque by IVUS with iMap analysis may predict slow flow during PCI.
Introduction
Percutaneous coronary intervention (PCI) is a common strategy for treating ischaemic heart disease, but it sometimes is complicated by the slow flow phenomenon, especially in patients with acute myocardial infarction (AMI)1-3. It is well known that the slow flow phenomenon is associated with adverse outcomes4,5. Various mechanisms are responsible for this phenomenon and several studies have evaluated predictors and treatments for slow flow during PCI6-10. However, the type of plaque associated with slow flow during PCI is unclear. Greyscale intravascular ultrasound (IVUS) is widely used to investigate coronary artery plaque. Several greyscale IVUS studies have shown that findings such as lipid pool-like images and positive remodelling are associated with slow flow during PCI11,12. However, conventional IVUS has limitations for assessing plaque composition13,14. Spectral analysis of IVUS data may be more useful for evaluating plaque because it allows detailed assessment of the components. Recently, several studies of virtual histology IVUS (VH-IVUS) (Volcano Therapeutics, Inc., Rancho Cordova, CA, USA) have demonstrated a correlation between plaque composition and the slow flow phenomenon15,16. However, it remains unclear which plaque components detected by VH-IVUS are associated with slow flow. iMap™-IVUS (iMap; Boston Scientific Corp, Fremont, CA, USA) is a new radiofrequency imaging system for tissue characterisation. iMap software converts the signal pattern into a frequency spectrum, and then compares it with spectra obtained from various tissues at autopsy (“data library”) to find the closest match. Since different tissue types (fibrotic, lipidic, necrotic and calcified) have distinctive spectra, iMap technology can identify tissues from the frequency spectrum that most closely resembles the one obtained by examination17. In this study, we investigated culprit plaque by iMap and examined whether tissue characterisation was effective for predicting the risk of slow flow after PCI.
Materials and methods
Study population
In this single-centre study, 95 consecutive patients underwent denovo PCI and iMap examination for ischaemic heart disease from July 2009 to January 2010. Of these, 33 patients had acute coronary syndrome (ACS). Exclusion criteria were tortuous vessels that precluded IVUS examination, a history of prior PCI, and other patients in whom adequate IVUS images could not be obtained. All patients were treated by stent implantation. Written informed consent was given by all patients.
PCI procedure
The patients received pre-medication with aspirin (200 mg/day) plus clopidogrel (75 mg/day) or 300 mg of clopidogrel as a loading dose. They also received intravenous unfractionated heparin just before PCI to achieve an activated partial thromboplastin time >250 sec. None of the patients were given glycoprotein IIb/IIIa inhibitors because these drugs have not been approved in Japan. If thrombus was detected by angiography, percutaneous thrombectomy was performed with an aspiration catheter to minimise the influence of the thrombosis on subsequent IVUS examination. If the IVUS catheter could not cross the lesion because of severe stenosis, dilatation was performed with a small balloon before advancing the catheter. If coronary flow was decreased on angiography after PCI, the patient was given an intracoronary injection of nicorandil (2-4 mg).
Definitions
Slow flow was defined as thrombolysis in myocardial infarction (TIMI) grade 0, 1 or 2 flow on angiography during the procedure with no evidence of spasm or dissection.
IVUS imaging and analysis
IVUS was performed before stenting and after intracoronary administration of 125-250µg of nitroglycerine. Data were acquired with a 40MHz IVUS catheter (Atlantis SR Pro; Boston Scientific Corp, Fremont, CA, USA). The catheter was advanced beyond the target lesion, and imaging was performed during automatic pullback at a speed of 0.5 mm/sec. The IVUS data were stored on a hard disk for off-line analysis, which was performed independently by experienced analysts who were unaware of the angiographic findings or the baseline clinical and lesion characteristics. Quantitative analysis of greyscale IVUS images was performed according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on IVUS18. Qualitative analysis of culprit lesions was performed with iMap software (QIvus 2.0; Medis medical imaging systems bv, Leiden, The Netherlands). Manual outlining of the lumen and the media-adventitia interface was performed for each culprit lesion, which was defined as the segment treated by PCI after IVUS examination. iMap software-classified plaque into four tissue components and produced colour images (green for fibrous plaque, yellow for lipidic plaque, red for necrotic plaque and blue for calcified plaque). The absolute volume (mm3) and percentage of each tissue component were determined. Plaque that was unsuitable for analysis because of acoustic shadowing behind calcification or wire artefact was removed automatically.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) for Windows version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Continuous variables were expressed as the mean ± standard deviation, while discrete variables were presented as numbers and percentages. Differences of mean values between groups were assessed by the unpaired Student’s t-test. Receiver-operating characteristics (ROC) analysis was employed to determine the optimal cut-off value for the percentage or absolute volume of each plaque component that predicted angiographic slow flow, with the area under the curve (AUC) being calculated as a measure of accuracy. In all analyses, p<0.05 was taken to indicate statistical significance.
Results
Patient profile
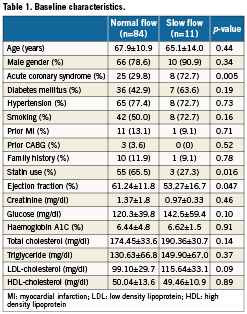
The subjects were divided into two groups, which were patients with slow flow and patients with normal flow after PCI. Among the 95 patients, slow flow was observed in 11 patients (11.6%). The baseline characteristics of the two groups are summarised in Table1. Patients with slow flow had a significantly higher prevalence of acute coronary syndrome (72.7% vs.29.8%, p=0.005), and significantly lower use of statins (27.3% vs. 65.5%, p=0.016). There were no significant differences between the two groups in terms of age, gender, and traditional coronary risk factors.
Angiographic findings
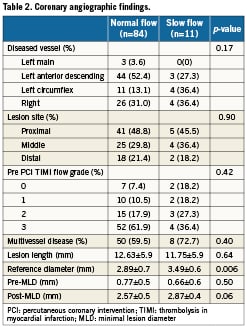
Coronary angiographic findings are summarised in Table 2. There were no significant differences of the vessels involved, culprit lesions, and pre-PCI TIMI flow grades. Minimum lesion diameters before and after PCI were also similar in both groups. However, the reference vessel diameter was significantly larger in the slow flow group (3.49±0.6 mm vs. 2.89±0.7 mm, p=0.006).
IVUS findings
IVUS data are summarised in Table 3. Greyscale IVUS analysis showed no significant differences of lesion length, luminal volume, and the percent plaque burden. However, the slow flow group had a larger vessel volume (281.2±150.7mm3 vs. 171.77±91.1mm3, p=0.0009) and absolute plaque volume (201.77±112.3mm3 vs. 121.89±67.4mm3, p=0.001) than the normal flow group. iMap analysis showed that there were no significant differences between the groups with respect to the percentage of fibrous plaque, lipidic plaque and calcified plaque. However, the percentage of necrotic plaque was significantly larger in the slow flow group than the normal flow group (19.73±5.1% vs. 14.56±8.3%, p=0.047). In addition, the slow flow group had a significantly larger absolute necrotic plaque volume (43.33±33.5 mm3 vs. 20.08±17.2 mm3, p=0.0004), fibrotic plaque volume (114.43±67.1mm3 vs. 75.04±41.1mm3, p=0.007), and lipidic plaque volume (14.12±8.1 mm3 vs. 8.11±5.5mm3, p=0.002) than the normal flow group. There was also a significant difference of absolute calcified plaque volume between the groups. Representative examples of iMap-IVUS images in patients with or without slow-flow phenomenon are shown in Figure 1.
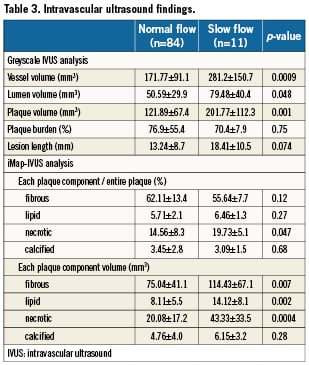
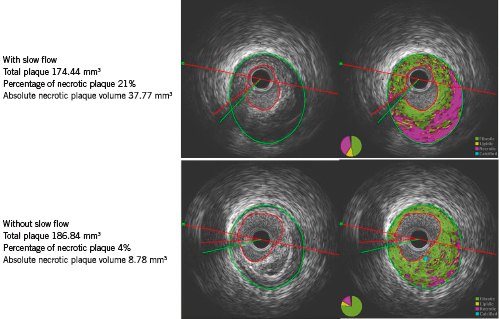
Figure 1. Representative examples of greyscale and i-Map intravascular ultrasound images in patients with or without the slow flow phenomenon.
ROC analysis
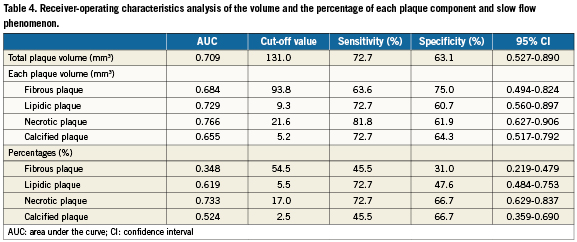
We performed ROC analysis to determine the diagnostic accuracy of each plaque component for predicting the slow flow phenomenon (Figure 2). Table 4 displays the results of ROC analysis of total plaque volume, the absolute volume of each plaque component and the percentage of each plaque component. Total plaque volume and the percentage of necrotic plaque both clearly predicted the occurrence of slow flow after PCI. However, the AUC for the percentage of necrotic plaque was larger than that for total plaque volume. In addition, the AUC for absolute necrotic plaque volume was 0.766 and was the largest among the variables assessed. The optimum cut-off value for prediction of slow flow from the absolute necrotic plaque volume was 21.6 mm3, showing a sensitivity of 81.8% and a specificity of 61.9%
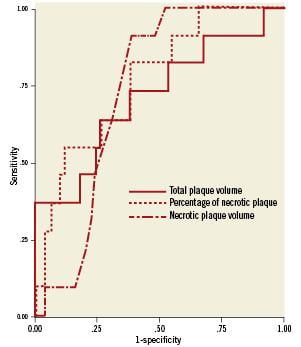
Figure 2. Receiver-operating characteristics (ROC) curves for the prediction of slow flow phenomenon. ROC curves demonstrated that the best predictive variables of IVUS findings for slow flow phenomenon was necrotic plaque volume detected by iMap-IVUS.
Discussion
The present study demonstrated that analysis of IVUS data for tissue characterisation with iMap software could predict the slow flow phenomenon during PCI. In this study, the slow flow phenomenon was not only related to total plaque volume, but also to the percentage of necrotic plaque. Therefore absolute necrotic plaque volume was the most related to the slow flow phenomenon during PCI. This shows that qualitative analysis of plaque is important like qualitative analysis. ROC analysis revealed that the most reliable IVUS parameter for prediction of slow flow was the absolute necrotic plaque volume. When the cut-off value for absolute necrotic plaque volume was set at 21.6mm3 it had a sensitivity of 81.8% and a specificity of 61.9% for predicting slow flow.
Several mechanisms have been postulated to explain the slow flow phenomenon19,20. In recent studies using distal protection devices, release of plaque debris by PCI has been shown to result in distal embolisation21,22. A previous study using greyscale IVUS revealed that a heavier atherosclerotic plaque burden was correlated with distal embolisation in patients who had stable or unstable angina23,24. In other studies, it has been found that lipid-poor-like plaque, positive remodelling, ruptured plaque, ultrasonic attenuation, and spotty calcification are risk factors for slow flow in patients with acute coronary syndrome11,12,25,26. However, it is still unclear which characteristics of plaque lead to slow flow after PCI. In the present study, total plaque volume on greyscale IVUS was related to the slow flow phenomenon, but the percentage and absolute volume of necrotic plaque were more reliable predictors of slow flow. This suggests that for prediction of slow flow it is important to not only perform quantitative assessment of plaque with greyscale IVUS but also to assess plaque tissue characteristics.
VH-IVUS can provide both qualitative and quantitative information. There have already been reports about assessment of plaque by VH-IVUS, and characterisation of plaque components by this method might be useful to predict slow flow15,16, although the correlation of each plaque component with the slow flow phenomenon remains controversial. It is unclear whether fibrotic and fibrofatty plaque or the necrotic core is more relevant to slow flow27,28. Radiofrequency IVUS systems automatically detected the coronary vessel and lumen boundaries; however in order to generate reliable volumetric data, these automatically detected contours have to be visually checked and in majority of cases manually corrected29. As this is relatively time-consuming process. Plaque analysis with iMap has the function of automated trace assist, and with this function, it can provide volume, area and percentage of measurement quickly and easily. In most cases, this process does not take much time.
In the present study, we employed a higher frequency IVUS catheter than that used for VH-IVUS, which allowed plaque composition to be analysed in more detail.
It is well known that slow flow is associated with a worse outcome4,5, so prevention of this phenomenon is important. Distal protection devices may be able to prevent slow flow after PCI by avoiding distal embolism, but the efficacy of distal protection remains controversial. Routine use of a distal protection device is not yet recommended, but a device should probably be used if there is a high risk of slow flow30,31. Therefore, it is important to accurately predict the risk of slow flow before performing PCI.
Clinical implications
We used iMap software to evaluate the plaque components that were associated with slow flow after PCI. The best predictor of slow flow was the absolute necrotic plaque volume and a cut-off value of 21.6 mm3 had a sensitivity of 81.8% and a specificity of 61.9% for predicting slow flow. Use of distal protection devices could be effective in patients with a high risk of slow flow based on their absolute necrotic plaque volume.
Limitations
This was a retrospective study performed at a single centre with a relatively small sample size, so selection bias cannot be excluded. The prevalence of acute coronary syndrome differed between the patients with and without slow flow, and this affected statin use, plaque composition, and occurrence of the slow flow phenomenon itself. In our analysis, acoustic shadowing behind calcification or wire artefact which makes the analysis incomplete was removed automatically. Thus, the location of the guidewire and IVUS catheter may influence the result of plaque analysis. Even though all patients underwent thrombectomy with an aspiration catheter, the presence of residual thrombus in culprit lesions might have influenced the plaque composition data because iMap software cannot determine the presence of thrombus.
Conclusions
Tissue characterisation of plaque by iMap analysis of IVUS data may predict the risk of slow flow during PCI.
Conflict of interest statement
The authors have no conflict of interest to declare

