Suboptimal post-percutaneous coronary intervention (PCI) fractional flow reserve (FFR) values have been related to target vessel failure1. In the randomised FFR REACT trial, intravascular ultrasound (IVUS)-guided PCI optimisation in patients with a post-PCI FFR <0.90 significantly improved post-PCI FFR values2. The aim of this prespecified subanalysis was to assess the impact of post-PCI IVUS findings in addition to FFR pullback data on operator-defined optimisation strategies, assessed through a dedicated questionnaire.
The rationale and design of the FFR REACT trial have been published previously2. In brief, patients with an angiographically successful PCI and a post-PCI FFR <0.90 were randomised (1:1) to either IVUS-guided optimisation or no further treatment (the control arm). In the present subanalysis, only patients randomised to the IVUS-guided optimisation arm with complete IVUS imaging were included. The trial was approved by the local ethics committee, and all patients provided written informed consent.
Data on the optimisation strategy were routinely collected using a dedicated questionnaire directly after disclosure of the post-PCI FFR pullback data and before IVUS acquisition. To “would you perform additional treatment?”, the possible response options were 1) post-dilatation (of the initial stent), 2) additional stenting (without optimisation of the initial stent), 3) post-dilatation of the initial stent combined with additional stenting, or 4) no additional treatment. These results were compared to the final optimisation strategy performed after careful post-PCI IVUS evaluation. Statistical analyses were carried out using SPSS statistics version 28.0 (IBM), and a two-sided p-value <0.05 was considered statistically significant.
A total of 145 patients (152 vessels) were randomised to the IVUS-guided optimisation arm, of which 136 patients (143 vessels) had complete IVUS imaging. The median age was 66 (25th-75th percentile 59-72) years, and 86.0% of patients were male. A total of 73.4% of vessels were left main or left anterior descending coronary arteries (LM/LAD). The mean post-PCI FFR after angiographically successful PCI (before additional optimisation) was 0.83±0.05 and ranged from 0.56 to 0.89 (25th-75th percentile 0.80-0.87). According to the questionnaire, based on the FFR pullback data, operators would have performed additional optimisation in 81/143 (56.6%) vessels. Post-dilatation was considered in 38/143 (26.6%) vessels, additional stenting (without optimisation of the initial stent) in 42/143 (29.4%) vessels, and post-dilatation of the initial stent combined with additional stenting in 1/143 (0.7%) vessels (Figure 1A). Following IVUS evaluation, post-dilatation was performed in 49/143 (34.3%) vessels, additional stenting in 24/143 (16.8%) vessels, and post-dilatation of the initial stent combined with additional stenting in 25/143 (17.5%) vessels (Figure 1A).
Overall, the optimisation strategy was altered in 76/143 (53.1%) vessels after IVUS evaluation as compared to the planned treatment strategy based on FFR pullback data alone (p<0.001) (Figure 1B). More specifically, 32/62 (51.6%) vessels that were considered for conservative treatment based on FFR pullback findings received optimisation following IVUS evaluation (resulting in a limited increase in the post-PCI FFR: ΔFFR 0.015±0.060; p=0.13), whereas 15/81 (18.5%) vessels that were originally scheduled to receive optimisation were deferred from optimisation based on the post-PCI IVUS findings. Of note, concordance between FFR and IVUS with respect to any post-dilatation and any stenting was 69.2% and 72.1%, respectively (Supplementary Table 1).
Finally, the frequency of an altered optimisation strategy by IVUS analysis did not differ between LM/LAD vessels and non-LM/LAD vessels (53.3% vs 52.6%, respectively; p=0.94) (Supplementary Table 2).
Our findings demonstrate that, within the FFR REACT trial, IVUS evaluation significantly changed the final optimisation strategy applied in patients with a post-PCI FFR <0.90 as compared to what would have been done based on post-PCI FFR pullback data alone. As such, the proportion of cases within our study in which PCI optimisation was performed (98/143, 68.5%) was substantially higher compared to previous studies reporting on PCI optimisation based on FFR (pullback) data alone (34.5%-43.0%)34.
To date, a significant body of evidence related to intracoronary imaging has contributed to clear recommendations for achieving optimal PCI5. Conversely, practical and validated cut-off values for optimisation based on post-PCI physiological pullback data alone are scarce, illustrating the need for future studies addressing the relationship between post-PCI FFR, intravascular imaging findings, additional optimisation and improved patient outcome.
Limitations of the present analysis include the absence of motorised FFR pullback data and use of the recently introduced pullback pressure gradient, precluding any discrimination between (physiologically defined) focal and diffuse disease. In addition, there were no predefined cut-offs for FFR-based optimisation. Finally, post-PCI FFR measurements were performed with a microcatheter, which could have led to slightly lower post-PCI FFR values.
To conclude, in patients with a post-PCI FFR <0.90, findings from post-PCI IVUS imaging alter the optimisation strategy in more than 50% of cases as compared to a treatment strategy based on post-PCI FFR pullback data alone. Ongoing follow-up of the FFR REACT trial and future dedicated studies should further shed light on whether imaging-guided PCI optimisation improves outcomes in patients with a post-PCI FFR <0.90.
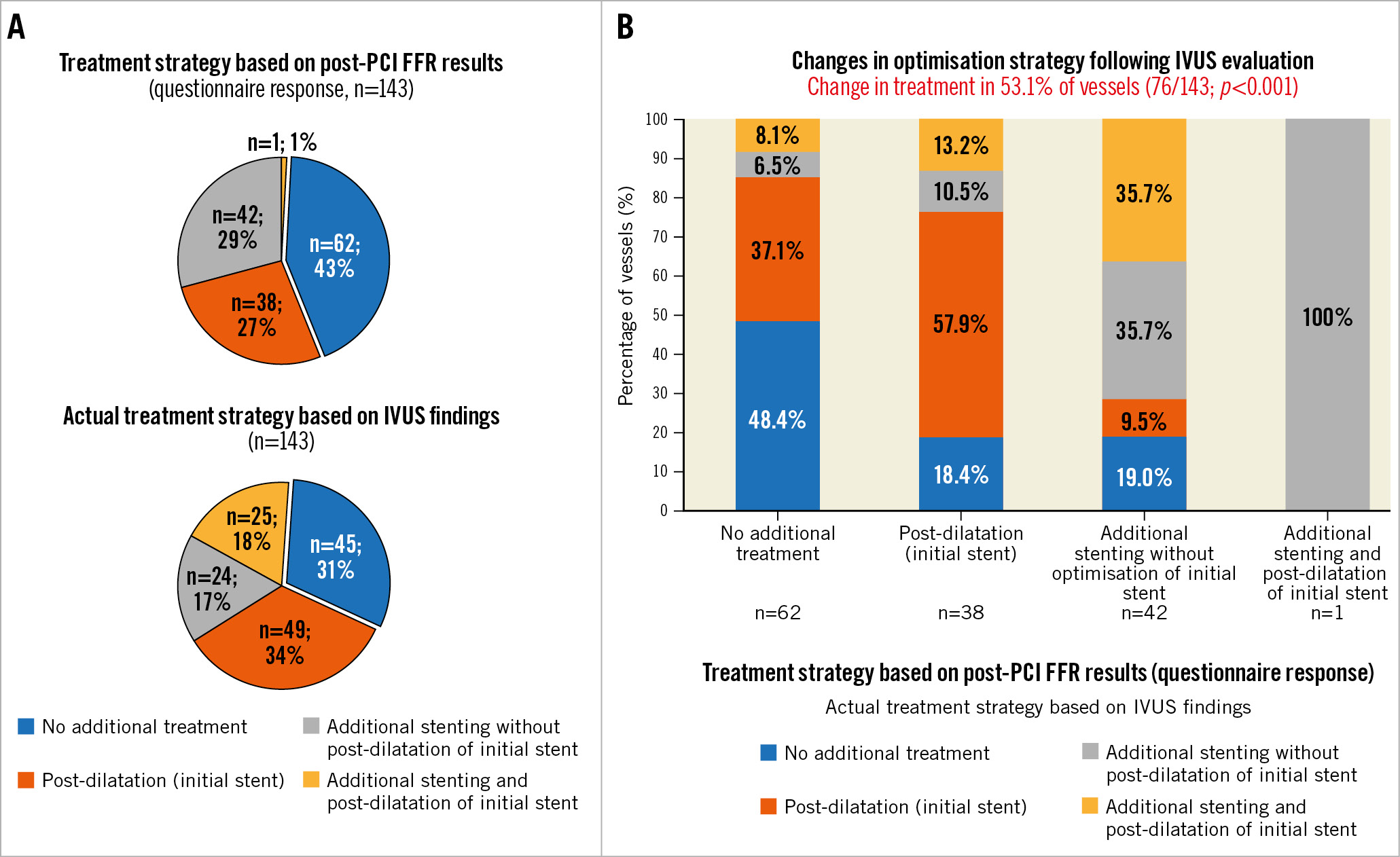
Figure 1. Change in treatment strategy after IVUS evaluation. A) The treatment strategies according to post-PCI FFR (questionnaire answers, upper pie chart) and the actual treatment strategy based on IVUS findings (lower pie chart). B) The proportion of vessels in which the treatment strategy was altered after IVUS evaluation, stratified by the proposed post-PCI FFR treatment strategy. FFR: fractional flow reserve; IVUS: intravascular ultrasound; PCI: percutaneous coronary intervention
Funding
This work was funded by institutional research support from ACIST Medical Systems, Inc.
Conflict of interest statement
J.Daemen received institutional grant/research support from AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical Systems, Medtronic, Pie Medical, and ReCor Medical; and consultancy and speaker fees from Abbott Vascular, Abiomed, ACIST Medical Systems, Boston Scientific, PulseCath, Pie Medical, Siemens Healthcare, and Medtronic. N.Van Mieghem received institutional research grant support from Abbott Vascular, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, and Daiichi Sankyo; and consultancy fees from Abbott, Boston Scientific, Medtronic, Abiomed, PulseCath BV, Daiichi Sankyo, Teleflex, and Amgen. T.Neleman received institutional grant support from ACIST Medical Systems. R.Diletti received institutional grant support from ACIST Medical Systems. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

