CASE SUMMARY
BACKGROUND: A 55-year-old male presented with acute anterior ST-elevation myocardial infarction (STEMI). Urgent coronary angiography revealed a subcritical stenosis on the proximal left anterior descending (LAD) and periapical occlusion of the LAD and distal part of its first diagonal branch which were successfully treated with balloon angioplasty and abciximab infusion. An intravascular ultrasound (IVUS) study performed three days later showed the presence of a huge plaque at the level of the proximal LAD.
INVESTIGATION: Physical examination, electrocardiography, laboratory tests, echocardiography, coronary angiography, intravascular ultrasound and virtual histology.
DIAGNOSIS: Significant proximal LAD stenosis with large plaque burden.
MANAGEMENT: Percutaneous coronary intervention.
KEYWORDS: acute myocardial infarction, intravascular ultrasound, percutaneous coronary intervention
PRESENTATION OF THE CASE
In September 2013, a 55-year-old male without previous medical history presented to the emergency department of another institution with chest pain and a mild increase of serum biomarkers of myocardial injury (troponin T 1.25 ng/dl). He was previously extremely fit and well, and was a non-smoker.
Following the administration of a loading dose of clopidogrel (600 mg) and acetylsalicylic acid (300 mg) he was taken to the catheterisation laboratory for an urgent coronary angiography with demonstration of a left anterior descending (LAD) intramyocardial coronary course (Figure 1A). Left circumflex and right coronary arteries were normal. The patient was discharged on aspirin.
Three weeks later he was admitted to our hospital because of an acute anterior STEMI complicated by ventricular fibrillation. He underwent urgent coronary angiography with demonstration of a subcritical stenosis (<50%) on the proximal segment of the LAD (Figure 1B) and periapical occlusion of the LAD and distal part of its first diagonal branch (Figure 1C, Moving image 1), which were successfully managed with a bolus administration of abciximab and balloon angioplasty (Figure 1D) since thromboaspiration was not considered an option because of the distal site of the occlusions.
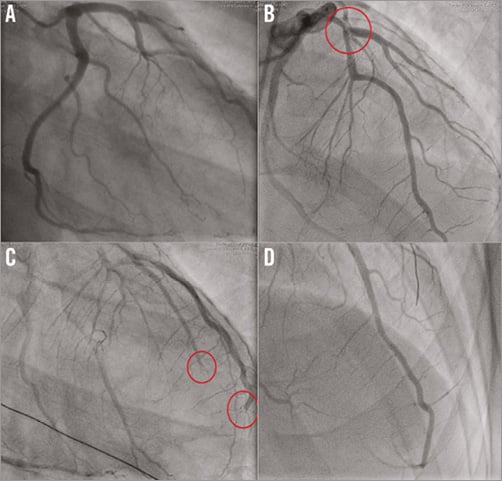
Figure 1. Coronary angiography. A) Left coronary artery in 30° right anterior projection. B) LAD coronary artery in cranial view: subcritical stenosis in proximal part (circle). C) Distal LAD and first diagonal branch occlusions. D) Primary PCI final result.
Echocardiography showed reduced contractility of the left ventricle (ejection fraction 40%) with akinesia of the apex and hypokinesia of the anterior-lateral wall. Contrast echocardiography was negative for right to left shunt both at rest and after Valsalva’s manoeuvre. Thrombophilic, autoimmune and toxicology screenings were negative.
Three days later the patient underwent a second coronary imaging study to analyse the left coronary artery with intravascular ultrasound (IVUS) and virtual histology (VH).
The IVUS-VH examination was performed with a dedicated IVUS-VH console (Volcano Therapeutics, Rancho Cordova, CA, USA) after intracoronary administration of 200 mg nitroglycerine. A 20-MHz, 2.9 Fr monorail, electronic Eagle Eye® Gold IVUS catheter (Volcano Therapeutics) was advanced into the LAD coronary artery after wiring, and automatic pullback at 0.5 mm/s was carried out to the aorto-ostial junction.
IVUS-VH uses spectral analysis of IVUS radiofrequency data to construct a tissue map. Qualitative and quantitative analyses of greyscale IVUS images were performed according to the criteria of the American College of Cardiology’s Clinical Expert Consensus Document on IVUS1.
At the level of the proximal LAD a “soft” coronary plaque was detected with a lesion minimal lumen area (MLA) of 5.9 mm2, an external elastic membrane (EEM) area of 31.3 mm2, an atheroma area of 25.5 mm2 and a plaque burden of 81.3% (Figure 2A). IVUS-VH investigation showed a so-called “pathological intimal thickening” (PIT), that is a mixture of all plaque components but predominantly fibrofatty plaque, with <10% confluent necrotic core and <10% confluent dense calcium according to PROSPECT definitions2 (Figure 2B, Moving image 2).
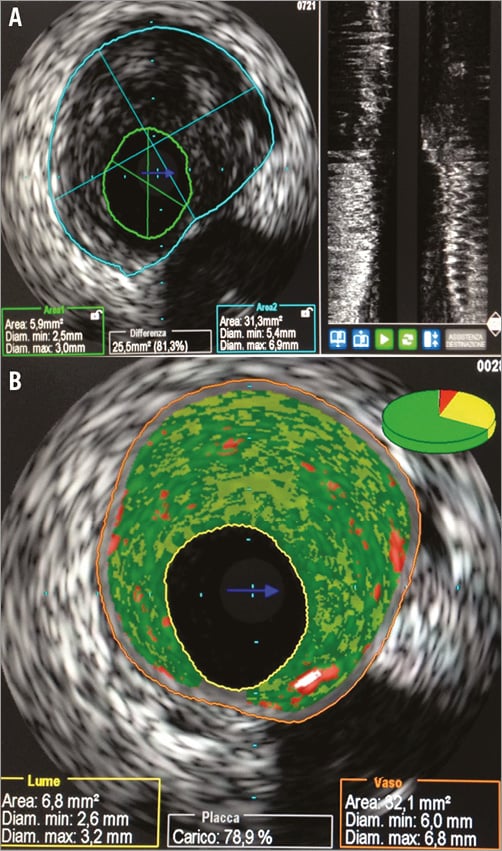
Figure 2. IVUS/virtual histology. A) Proximal LAD: “soft” coronary plaque (MLA 5.9 mm²; EEM area 31.3 mm²; plaque burden 81.3%). B) Proximal LAD: at IVUS-VH interrogation evidence of a so-called “pathological intimal thickening” (PIT).
How should we deal with this bifurcation lesion with a high risk of no-reflow because of clinical (repeated embolisations) and morphological (echolucent/large plaque burden) features?
How would I treat?
THE INVITED EXPERT’S OPINION
This is indeed a challenging case for at least two reasons. First, the patient had already presented with an acute myocardial infarction caused by embolisation, probably from this coronary segment. Thus, the risk of further embolisation during any percutaneous treatment persists. Secondly, plaque involves a bifurcation of a relatively large diagonal branch that should not be missed. Before deciding on the way to treat this lesion, one may question whether this non-significant stenosis (minimal lumen area of 5.9 mm2) induces ischaemia, and thus merits being treated. To demonstrate this, fractional flow reserve should be indicated. However, we have to take into account that this lesion was initially left untreated (only on oral medication) and the patient showed an acute recurrence. Thus, I view the indication of this case in the context of healing a high-risk plaque as part of a secondary prevention strategy to avoid further embolic events.
Once we decide to go for interventional treatment we may choose between CABG and PCI. In favour of CABG is the fact that the location of the lesion at the bifurcation does not infer any specific risk. However, two features may tip the scales towards PCI: the intramyocardial trajectory of the LAD and the fact that grafting two vessels with non-significant stenosis (<50%) may jeopardise the fate of the grafts due to competitive flow from the native arteries.
I would treat this lesion with the help of an embolic protection device (i.e., filter type) placed in the mid segment of the LAD. Additionally, I would place a protective wire in the diagonal branch. The bifurcation would then be treated by means of a provisional stenting technique. The stent would be directly implanted jailing the diagonal branch. In the event of any compromise at the ostium of the diagonal, I would re-cross a wire through the stent to the diagonal branch and perform kissing balloon inflation. In the event of distal embolisation of the diagonal, I would also re-cross a wire to the diagonal and try to perform manual thrombectomy with a small aspiration catheter. Finally, I would remove the wire from the diagonal and recapture the filter from the LAD. I would not use abciximab during this procedure as this had already been administered three days before.
The routine use of embolic protection devices in the setting of acute myocardial infarction has not been demonstrated in clinical trials3. However, a recent study has demonstrated that the presence of ruptured yellow plaque and of large plaque burden was highly predictive of distal embolisation of plaque debris4. In another study using near-infrared spectroscopy, it was demonstrated that plaques with large lipid core resulted more frequently in embolised material retrieved from the embolic protection device5. Our patient presented both conditions on virtual histology: large plaque burden and large fibrofatty content. For these reasons, I believe that the use of this device will be of help in this patient.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
This is a very educational case from which some teaching points should be discussed in a very careful way. Rapid progression of a localised lesion in the proximal left anterior descending (LAD) is multifactorial6,7, and overactivation of inflammation plays a critical role in the “progressive growth” of an already unstable plaque. Previous reports have demonstrated that cytokines (interlukin-1/-6) and chemokine (monocyte chemotactic protein [MCP]-1 and C-C motif ligand [CCL] 2) are overexpressed and phosphorylated, and are correlated with the plaque rupture, erosion and intraplaque haemorrhage, subsequently resulting in abrupt thrombus formation. Endothelial nitric oxide synthase (eNOS) “uncoupling” induces further endothelial injury and vascular constriction. From this point, measurement of inflammatory markers would be critical to assess the severity of an unstable plaque. Statin treatment started at a loading dose is recommended for such patients.
Another interesting finding is that the LAD and its first diagonal branch were occluded at the distal portions, but not at the site where the unstable progressive plaque was. In other words, the presence of multiple unstable plaque could be excluded in this case.
On the other hand, there is a discrepancy between anatomical parameters and functional significance in the coronary artery. For assessment of an intermediate coronary lesion, fractional flow reserve (FFR) demonstrates a unique advantage over intravascular ultrasound (IVUS), the latter usually overestimating the clinical significance of the intermediate lesions8. Studies have also indicated that the severity of non-culprit stenoses can reliably be assessed by FFR, even during the acute phase of an MI9,10. However, due to microvascular dysfunction, FFR is not recommended to assess the infarct-related artery during the acute setting of STEMI (<6 days)11. Obviously, functional assessment should be performed prior to making a decision on stenting the LAD in this patient.
Finally, there is a lack of data regarding the superiority of IVUS or virtual histology (VH)-IVUS over optical coherence tomography (OCT) in detecting intravascular thrombus. By IVUS, a thrombus is usually recognised as an intraluminal mass, often with a layered, lobulated, or pedunculated appearance12,13. However, the sensitivity/specificity of detecting thrombus is low, and the diagnosis of thrombus by IVUS should always be considered presumptive. Fresh thrombus usually appears as soft plaque. Up to now, there is no specific VH-IVUS algorithm to identify thrombus14,15. Thus, thrombus appears green or light green (fibrotic or fibrofatty plaque), and can be misclassified as pathological intimal thickening (PIT) by VH-IVUS. Due to its high spatial resolution (around 10 μm), OCT provides a unique advantage for detecting thrombus, even distinguishing red thrombus or white thrombus.
In conclusion, for this patient, early intensive anti-inflammatory and antiplatelet therapy after the first episode of an ischaemic attack might be mandatory to avoid the occurrence of later events. Assessment of the plaques by OCT would provide more information about the features of existing unstable plaques. FFR measurement is in line with current guidelines before making a decision on stenting any intermediate lesions. Dual antiplatelet therapy is no doubt mandatory for medication following coronary stenting procedures.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
It was decided that percutaneous intervention should be the therapeutic option for this case.
The patient was already on double antiplatelet therapy. After full anticoagulation with weight-adjusted unfractionated heparin, a 7 Fr XB 3.5 (Cordis, Johnson & Johnson, Warren, NJ, USA) guiding catheter was used to cannulate the left main via the right femoral artery.
The LAD was wired with an extra-support Iron Man wire (Abbott Vascular, Santa Clara, CA, USA) and the first diagonal branch with a Balance Middleweight wire (Abbott Vascular) because of relevant plaque burden despite the fact that the ostium of the side branch appeared free of disease on IVUS.
The decision was taken to perform direct stenting of the lesion in the LAD with a 22 mm self-expanding nitinol STENTYS 3.5-4.5 (STENTYS, Paris, France) over the ostium of the side branch. After adequate positioning (Figure 3A), the stent was delivered by retracting the covering sheath. Because of incomplete coverage of the lesion a second balloon-expandable bare metal stent, a 3.0×18 mm Integrity (Medtronic, Minneapolis, MN, USA), was deployed at 14 atm.
After STENTYS implantation, a tight stenosis (90%) was appreciated at the level of the diagonal branch (Figure 3B). For this reason, after rewiring of the diagonal branch through the stent wall in the cell closest to the carina, such that optimal scaffolding of the ostial side branch could be provided, disconnection of the strut overlying the ostium was performed with a Maverick 3.0×12 mm (Boston Scientific, Marlborough, MA, USA) inflated at 8 atm.
The final angiographic control showed a good result in the main vessel but the persistence of a suboptimal result at the level of the side branch: a 75% stenosis without images of dissection and with a TIMI III flow (Figure 3C, Moving image 3).
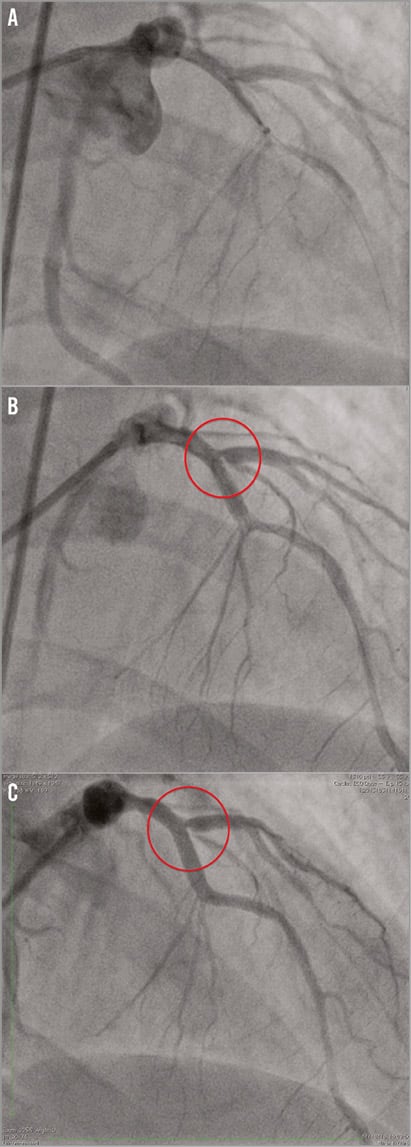
Figure 3. Coronary angiography. A) STENTYS positioning at the level of proximal LAD. B) Final result: tight stenosis at ostial first diagonal branch. C) First diagonal branch: suboptimal results after dilatation with a Maverick 3.0×12 mm at 12 atm.
An IVUS interrogation of the LAD showed an acceptable result at the level of the main vessel, with a well-apposed stent and a minimal stent area of 7.6 mm2 (Figure 4).
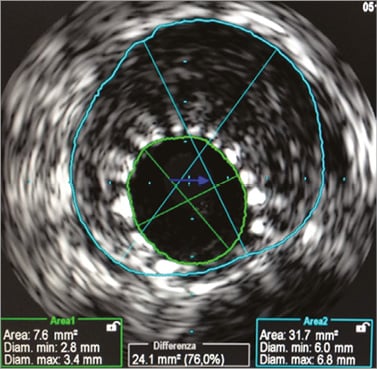
Figure 4. Proximal LAD: final result after STENTYS implantation.
In order to avoid the unnecessary deployment of an additional stent in the side branch with subsequent aggressive kissing balloon inflation, we decided to perform a functional evaluation of the LAD and the diagonal branch. A PrimeWire PRESTIGE® PLUS Pressure Guide Wire (Volcano Corp., Rancho Cordova, CA, USA) was calibrated and introduced into the guiding catheter, and digital haemodynamic data were extracted from the data storage system ComboMap. iFR was calculated as a ratio of the distal coronary pressure to proximal coronary pressure at rest, using the validated automated algorithms with phase alignment acting over the diastolic wave-free period over a minimum of five beats. Results of functional evaluations were as follows: iFR/LAD 1.0 and iFR/Diag. 0.96 (Figure 5).
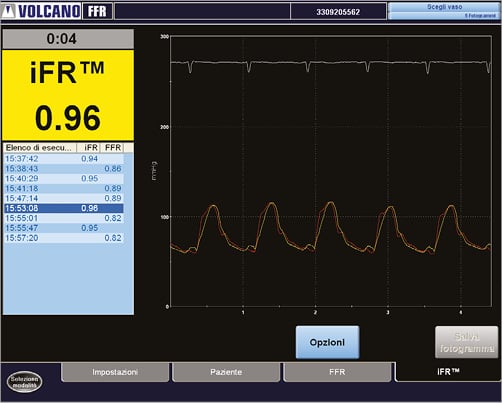
Figure 5. Functional evaluation of diagonal branch with iFR.
A decision to stop the procedure was taken. The patient did not suffer any major adverse cardiovascular event during the hospitalisation and was discharged two days later. Three months later, he was in good condition and free of angina.
Discussion
PCI in patients affected by acute coronary syndromes can predispose to stent thrombosis owing to the large thrombotic burden, suboptimal stent expansion, or stent insertion on a thrombotic milieu that eventually disappears leading to malapposition16. On the other hand, deployment of oversized stents and high-pressure inflations can cause plaque or thrombus disruption and distal embolisation with subsequent lack of myocardial salvage, with poor short- and long-term clinical outcomes17.
Mobilisation of thrombotic material and/or plaque debris during primary PCI, causing distal embolisation, has been suggested as a potential mechanism in the pathophysiology of no-reflow18,19. Angiographic signs of macroscopic distal embolisation have been reported to occur in up to 15% of patients undergoing primary PCI20.
Because of clinical (repeated embolisations) and IVUS data (very large plaque burden) we thought that the risk of distal embolisation and no-reflow was very high following the implantation of a balloon-expandable stent.
Theoretically, the risk of mobilising plaque debris or thrombotic fragments during stent implantation could be offset by a protection device, even though in STEMI trials this policy did not translate into an angiographic, enzymatic or clinical benefit21,22. However, this solution was not taken into consideration because of the necessity to protect both the LAD and the large diagonal branch.
In vessels with large plaque burden and/or a high thrombus load, the feature of self-expansion can potentially reduce the occurrence of stent malapposition and distal embolisation, and thus of stent thrombosis as well as no-reflow. For this reason we decided to implant a self-expandable stent.
The STENTYS® coronary stent is a nitinol self-expanding tubular stent, available in drug (paclitaxel) eluting and bare metal forms with a nominal strut width of 0.0027’’ (68 microns). A 6 Fr compatible, rapid-exchange delivery system delivers the stent into position over a conventional 0.014” guidewire, and the stent is deployed by the withdrawal of a retractable sheath. The stent has a Z-shaped design that is linked together by small interconnections which can be disconnected by balloon inflation between the struts to create side branch access, if needed.
The ability of this stent to grow in volume in the first hours to days after the procedure allows gentle deployment with less trauma, but also reduces plaque disruption or thrombus dislodgement and could thus lead to less distal embolisation. The increase in the STENTYS stent area (19%) at three days after implantation and the absence of malapposed stents seen at six months suggest that this device follows the growth of the vessel lumen while vasoconstriction and thrombus are resolving23.
Another interesting feature of this device is the presence of small distinctive interconnectors that can be used to create an opening through the stent. It allows the physician to deploy the stent safely, then disconnect the stent interconnectors with an angioplasty balloon to provide side branch access independent of the side branch ostium location. The interconnectors are placed all along the length and the circumference of the stent, apart from the first and last 2 mm. We thought we would take advantage of this characteristic perhaps eventually to treat the ostium of the large diagonal branch if needed: it is well known that the main predictors of side branch occlusion by volumetric IVUS are plaque volume decrease in the proximal main vessel and acute clinical presentation24.
As a matter of fact, the tight stenosis at the ostium of the diagonal branch improved only partially after single balloon inflation. For this reason, we decided to perform a functional evaluation of both the LAD and the first diagonal, since angiographic evaluation alone is frequently inaccurate and does not reflect the functional severity of ostial lesions25.
The instantaneous wave-free ratio (iFR) is a novel pressure-only invasive index of coronary stenosis severity which does not require the administration of vasodilator drugs, such as adenosine26. Recent studies which directly compared the classification of intermediate coronary stenoses by iFR and FFR26 revealed a consistent pattern of agreement with FFR.
In our patient we did not find a significant reduction in coronary reserve, despite the presence of a significant ostial stenosis at coronary angiography. We could therefore avoid implanting a new stent and performing an aggressive kissing balloon inflation.
Conclusions
In the presence of large embolising plaque, self-expandable stent implantation could be an interesting option in order to avoid the no-reflow phenomenon. The gentle expansion forces are particularly well suited for deployment in an artery with a heavy clot burden, such as in acute settings. A functional evaluation at the end of the procedure by means of iFR is the ideal companion to this strategy, since it avoids an unnecessary post-dilatation, a risky business in this lesion subset.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Distal LAD and first diagonal branch occlusions.
Moving image 2. Proximal LAD: at IVUS-VH interrogation evidence of a so-called “pathological intimal thickening” (PIT).
Moving image 3. First diagonal branch: suboptimal results after dilatation with a Maverick 3.0×12 mm at 12 atm..
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Distal LAD and first diagonal branch occlusions.
Moving image 2. Proximal LAD: at IVUS-VH interrogation evidence of a so-called "pathological intimal thickening" (PIT).
Moving image 3. First diagonal branch: suboptimal results after dilatation with a Maverick 3.0x12 mm at 12 atm..

