
Abstract
Aims: Selective use of DES only in patients at higher risk of MACE is common practice, particularly in healthcare systems with a large premium payable for DES. We aimed to identify subgroups of patients in which the use of BMS in primary percutaneous coronary intervention (PPCI) for STEMI can still be justified.
Methods and results: We performed a patient-level pooled analysis of COMFORTABLE AMI and EXAMINATION comparing contemporary DES with BMS in PPCI. A risk score was applied using three parameters: lesion length >15 mm, vessel size <3 mm, and diabetes mellitus. Individual data were available for 2,655 patients. The incidence of MACE at one year was incrementally higher in patients with risk scores of 1 or 2/3. MACE rates were lower in patients with a risk score 0 or 1 who were treated with DES (p=0.0073 and p=0.008). No difference in death or reinfarction was seen between DES and BMS in any group. There was a significant reduction in TLR with DES in all three groups.
Conclusions: A score comprising vessel size, lesion length, and diabetes did not identify patients at low risk with equivalent or better results from BMS use. The results suggest that the practice of only selective use of DES in primary PCI should be discouraged.
Introduction
Drug-eluting stents (DES) have been shown to reduce the risk of restenosis and related events when compared to bare metal stents (BMS)1-5. The benefit over BMS is marked in some patient subgroups and lesion subsets and is less pronounced or absent in others6-11. Use of multiple stents, vessel diameter and diabetes have long been identified as predictors of restenosis after stent implantation12. In 2003, the then National Institute of Clinical Excellence (NICE) in the United Kingdom issued guidance to use DES over BMS selectively in lesions longer than 15 mm and vessel diameters <3 mm. The recommendation was based on cost-effectiveness analyses of available data, was confirmed in a revision in 200813, and has not been revised since. Selective use of DES has become common practice, particularly in healthcare systems with limited resources. Often, additional patient factors such as the presence of diabetes and the mode of presentation are taken into account when choosing between DES and BMS14-16.
In primary percutaneous coronary intervention (PPCI) for acute ST-elevation myocardial infarction, first-generation DES have shown ambiguous results compared to BMS16-22. In the first year following implantation a reduced rate of TLR was documented; however, late stent thrombosis was seen more frequently in the following years and, overall, there was no benefit with a strategy of general use of DES23.
More recent results with newer-generation DES suggest a reduction in TLR, as well as reduced stent thrombosis, when DES are used in the treatment of STEMI. The COMFORTABLE AMI and EXAMINATION trials both reported improved outcomes in PPCI24,25. However, it remains unclear whether all patients presenting with STEMI should be treated with DES or whether differential use of DES and BMS can be justified.
It remains common practice to use DES selectively. In the recently published large-scale trials TASTE26 and TOTAL27, DES were used in less than half of the cases (47.5% and 45%). Given the large number of PPCI performed worldwide, the selective use of DES has an important cost impact.
We postulated that we would be able to identify patient subgroups at low risk of restenosis and MACE, in which DES would not have a clinically relevant benefit over BMS in PPCI.
Methods
Both COMFORTABLE AMI and EXAMINATION have been described in detail28,29.
Briefly, the multicentre COMFORTABLE AMI trial (NCT00962416) randomly assigned 1,161 patients to treatment with biolimus-eluting stents with biodegradable polymer and bare metal stents of otherwise identical design at 11 international sites. The primary endpoint was a composite of cardiac death, target vessel MI and target lesion revascularisation at one year.
The EXAMINATION trial (NCT00828087) was a multicentre, prospective, randomised, all-comer, controlled trial carried out in 12 medical centres in three countries. Patients with STEMI up to 48 hrs after the onset of symptoms requiring emergent percutaneous coronary intervention were randomly assigned (ratio 1:1) to receive an everolimus-eluting stent (EES) or a BMS. The primary endpoint was the patient-oriented combined endpoint of all-cause death, any recurrent myocardial infarction, and any revascularisation at one year, and was analysed by intention to treat. A total of 1,498 patients were randomly assigned to receive EES (n=751) or BMS (n=747).
The two trials had different primary endpoints. We used individually pooled data of adjudicated events, so we could align the events across the two studies.
Data set
Individual patient data were available for the COMFORTABLE and EXAMINATION trials.
Three risk criteria were defined based on the recommendations of NICE and previous analyses for differential benefits of DES over BMS12: 1) small target vessel diameter (<3.0 mm), 2) long lesion length (>15 mm) (for this, the maximum stent diameter at implantation and a stent length of ≥20 mm were used), 3) presence of diabetes mellitus.
The patients were then grouped according to the risk score: 0 (absence of the above risk factors, i.e., patients without diabetes, short lesion and large target vessel), 1 (presence of one risk factor), 2 (presence of two or more risk factors)30. The different outcomes in patients receiving either BMS or a drug-eluting stent (BES or EES) were analysed. Patients were stratified according to the randomised stent, i.e., by intention-to-treat.
Statistical analyses
Continuous data are presented as means (±SD, p-values from ANOVAs) and categorical data as counts (%, p-values from logistic regressions). Individual pooled analyses were used to assess differences in clinical outcomes comparing DES vs. BMS (intention-to-treat), within each of the three risk score groups, for the pre-specified device-oriented composite endpoint of cardiac death, target vessel infarction and clinically indicated target lesion revascularisation (MACE) at one year, and for the pre-specified endpoints of all-cause death, any infarction, clinically indicated target lesion revascularisation and definite stent thrombosis at one year. Cox’s regressions per endpoint, per risk group and per trial comparing DES vs. BMS for time-to-first event were used to derive effect sizes (stratified by trial when the two trials were pooled). Individual pooled Cox’s regressions were used to assess a linear trend over the risk groups comparing the randomised stents, again per endpoint, stratified per trial. Kaplan-Meier curves are based on the pooled individual data. As a sensitivity analysis, meta-analyses and meta-regressions were performed, using a continuity correction of 0.5 in case of zero events (Online Figure 1, Online Figure 2). A two-sided p-value <0.05 was considered as statistically significant. Statistical analysis was performed using Stata software, version 12.1 (Stata Corp., College Station, TX, USA).
Results
Individual data were available for 2,655 patients. The baseline characteristics are summarised in Table 1. The majority had one risk factor for adverse outcomes.
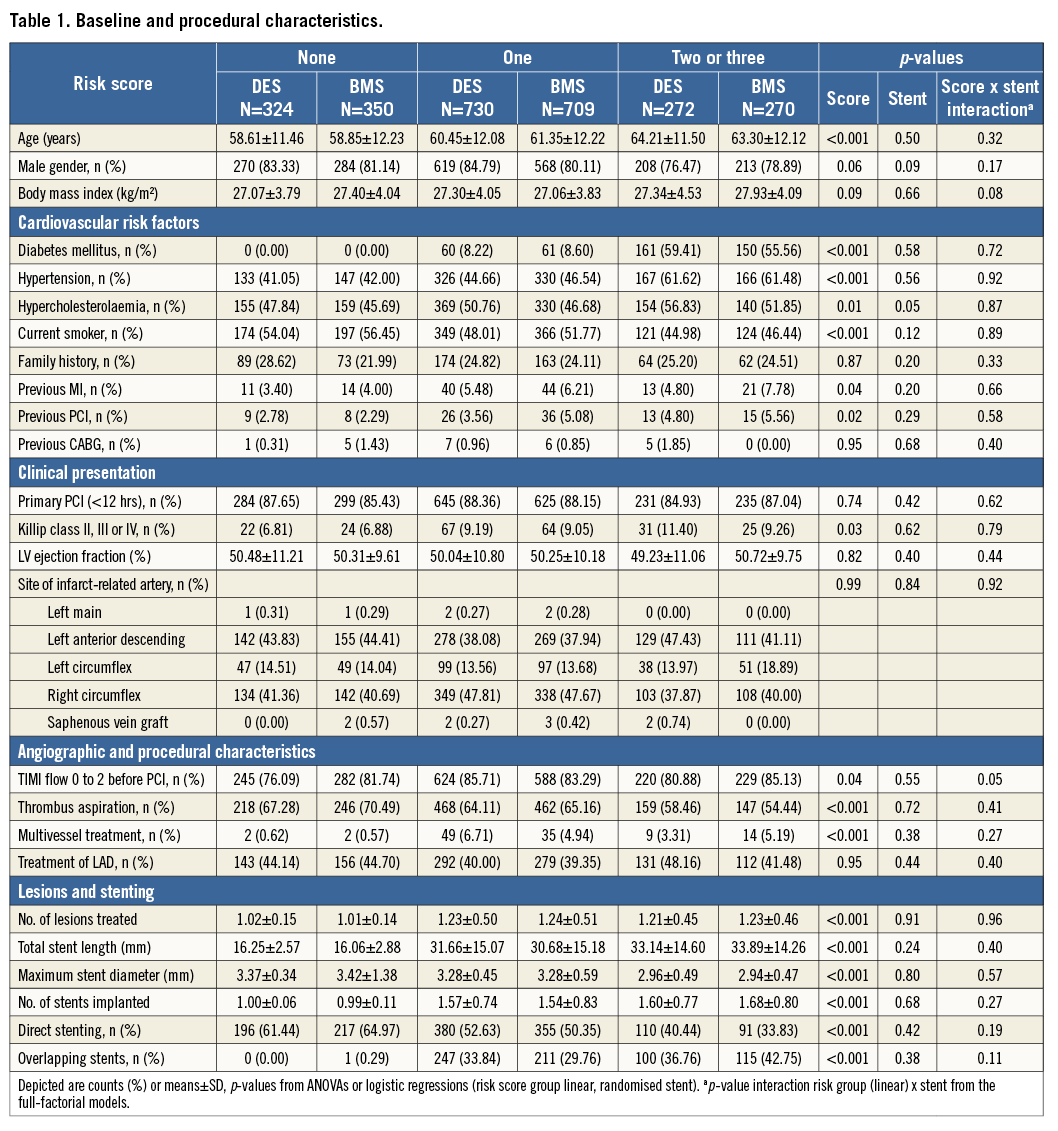
The groups with a risk score of 1 or more showed a significantly higher incidence of diabetes, hypercholesterolaemia and hypertension (p<0.01), therefore identifying a generally higher risk population for cardiovascular events.
The group with a risk score of 0 showed significantly shorter lesion length, larger stent size used, and higher use of direct stenting techniques (all p<0.001). On average in this group one stent was used for one lesion treated.
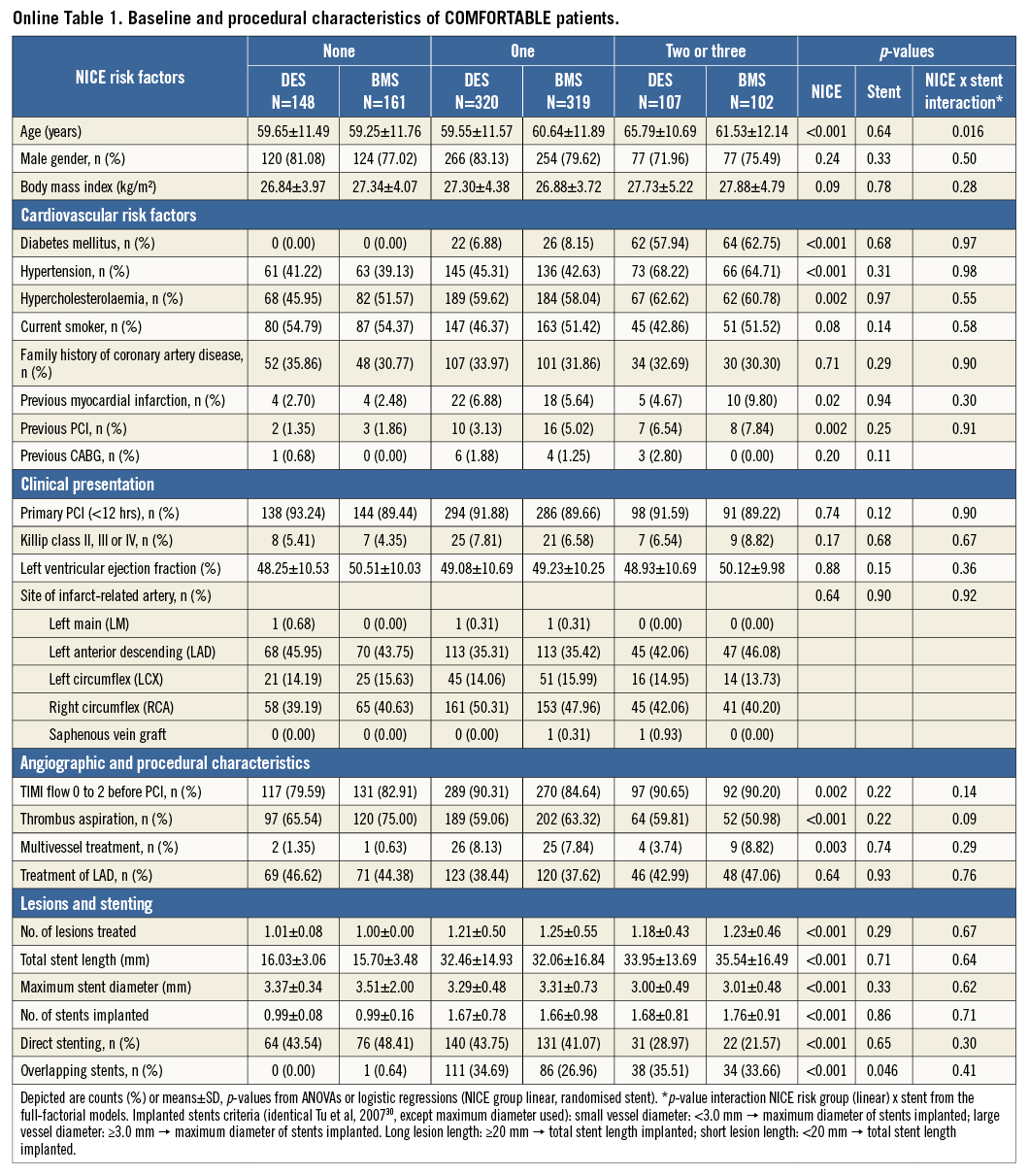
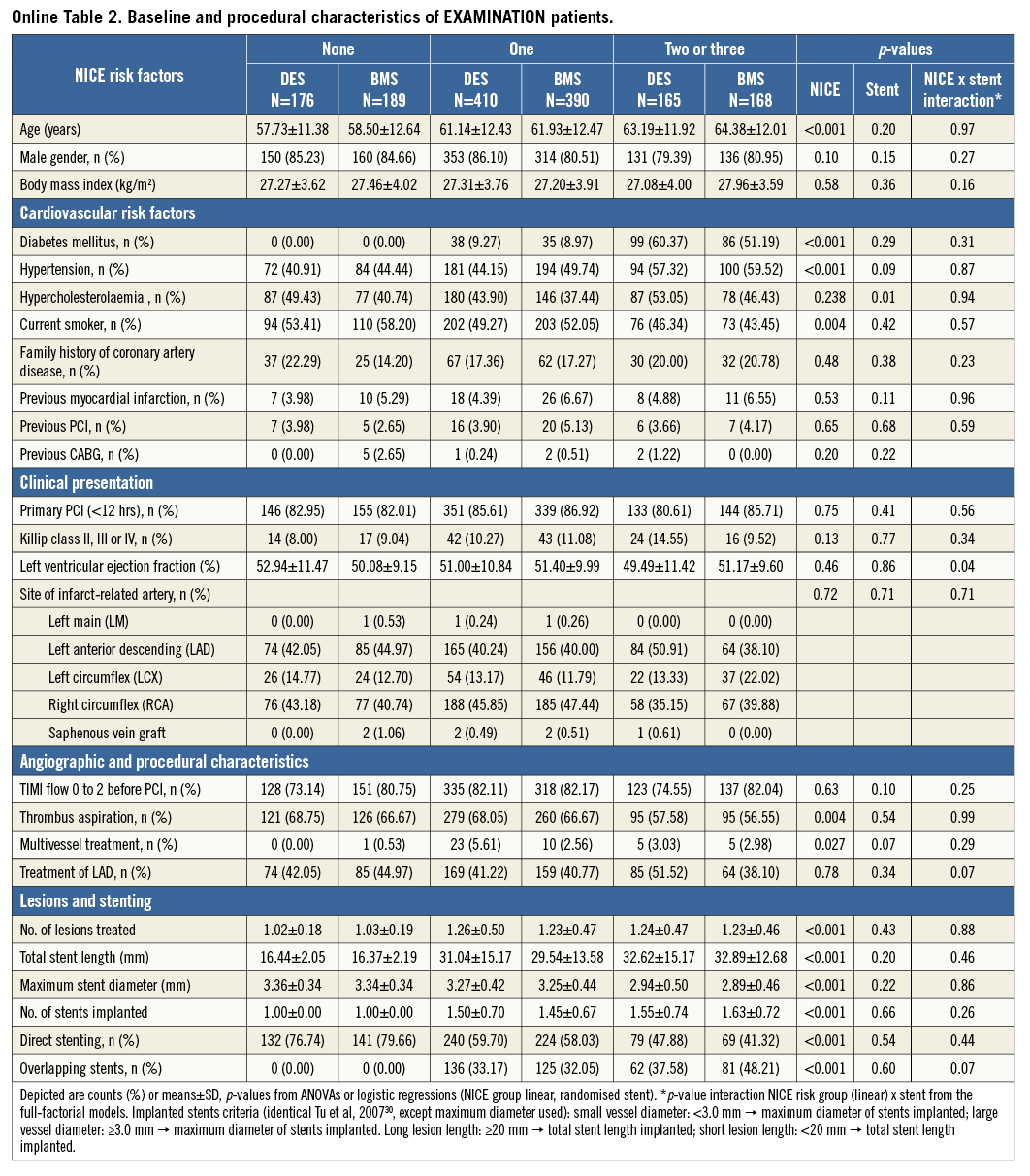
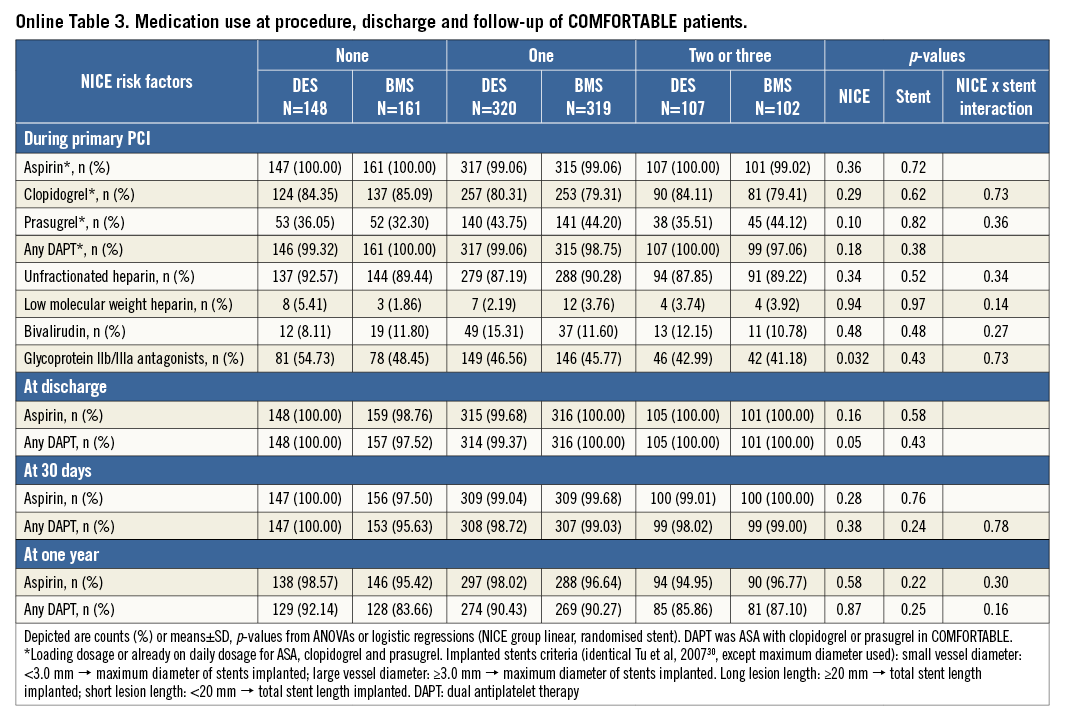
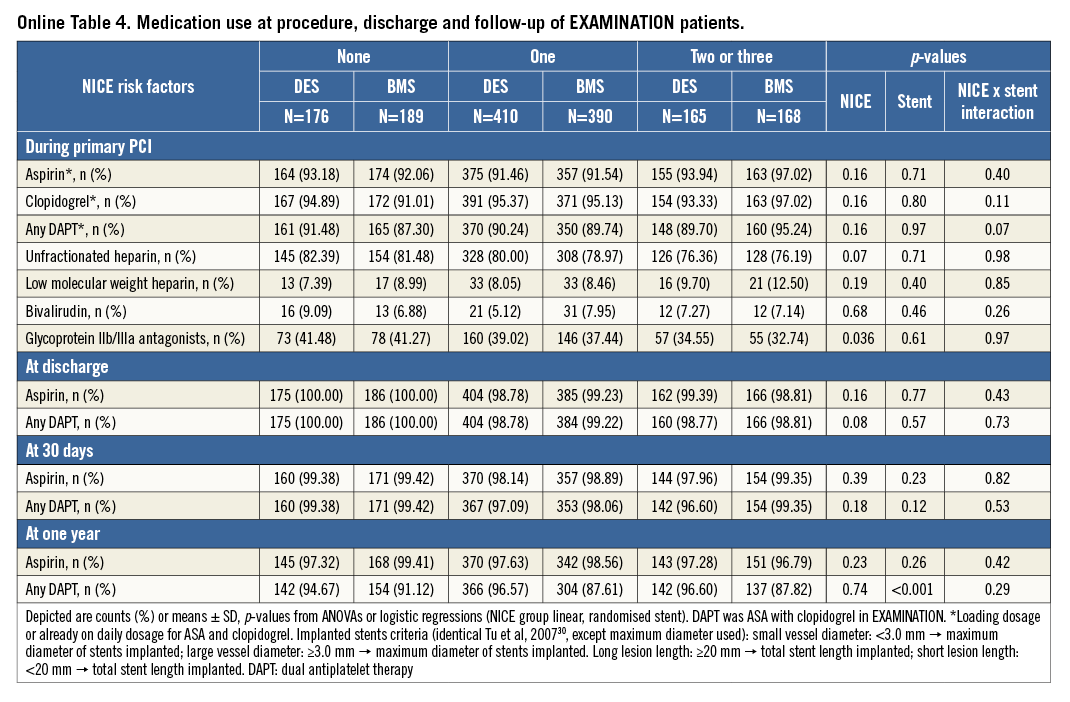
The pharmacologic treatment in both trials was at the discretion of the operator. In patients with a risk score of 0, more GP IIb/IIIa inhibitors were used (p<0.001) and a higher use of unfractionated heparin was reported (p=0.02). The details are illustrated in Table 2. Online Table 1-Online Table 4 are supplied to show the data sets of Table 1 and Table 2 separately for the two trials.
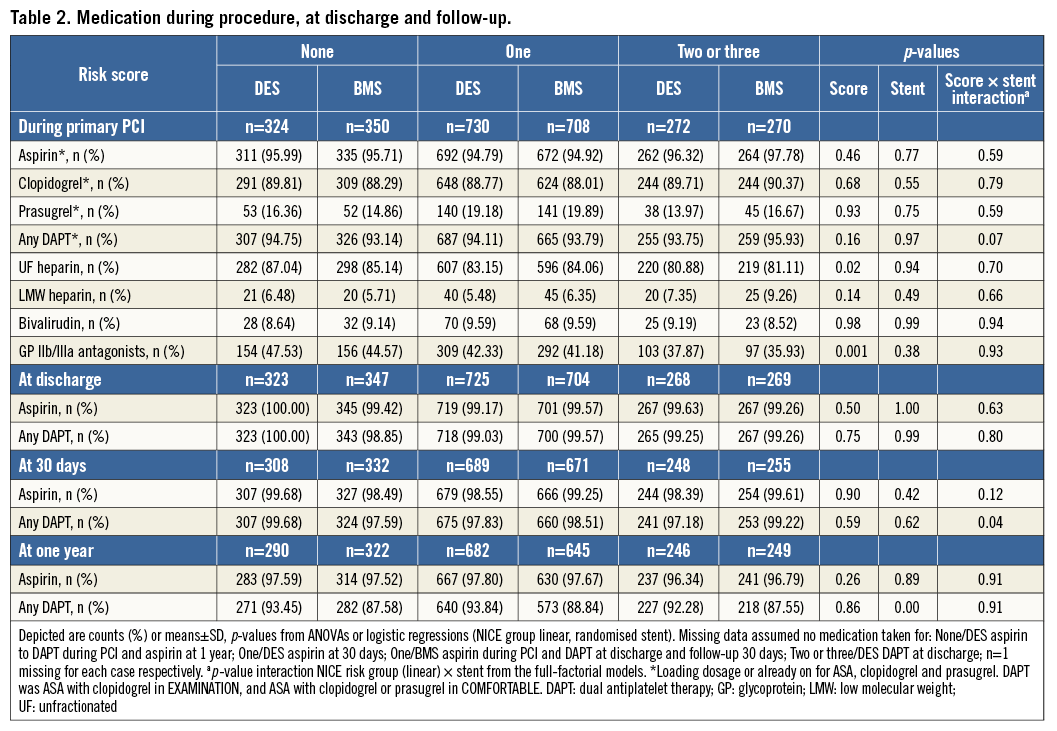
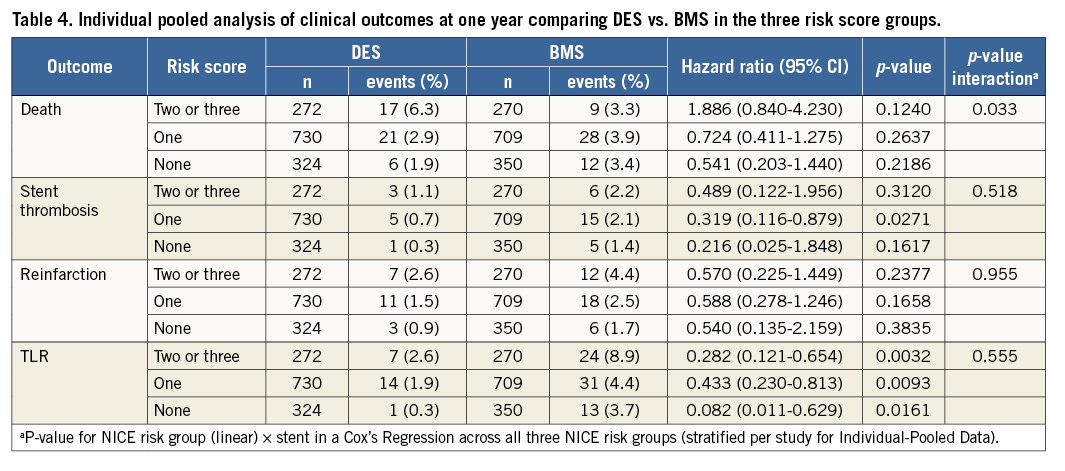
The incidence of MACE at one year was incrementally higher in patients with risk scores of 1 or 2 and 3. In COMFORTABLE AMI, the patients with risk scores of 0 or 1 treated with DES had a significantly lower MACE rate compared with patients treated with BMS (p=0.0009 for risk score 0 and p=0.035 for risk score 1).
In EXAMINATION, the rate of MACE was lower in patients treated with DES in all three groups; however, this difference was not statistically significant. The pooled analysis showed significantly lower MACE rates in patients with risk score 0 or 1 who were treated with DES (p=0.0073 and p=0.008). In patients with a risk score of 2 or 3, deemed at highest risk for restenosis and adverse cardiac events, the difference in MACE at one year between the patients treated with DES or BMS did not reach statistical significance. Details are shown in Table 3. Figure 1 provides Kaplan-Meier curves for MACE and revascularisation.
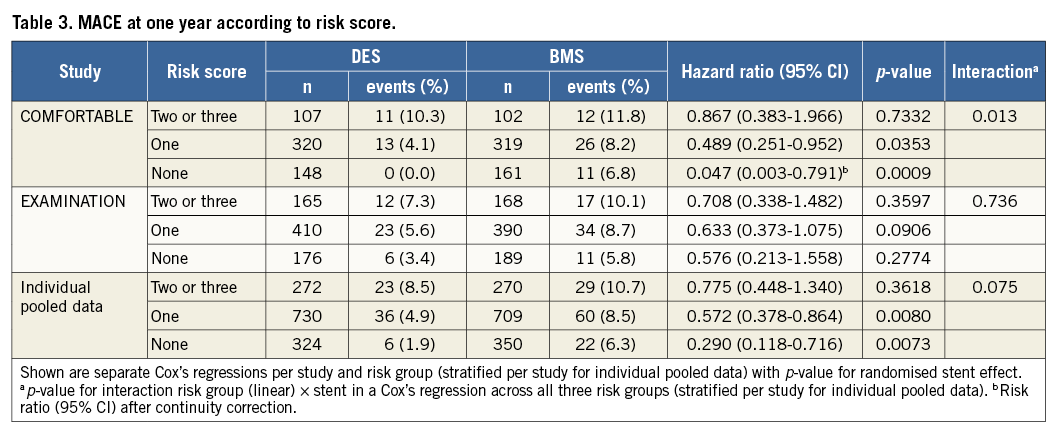
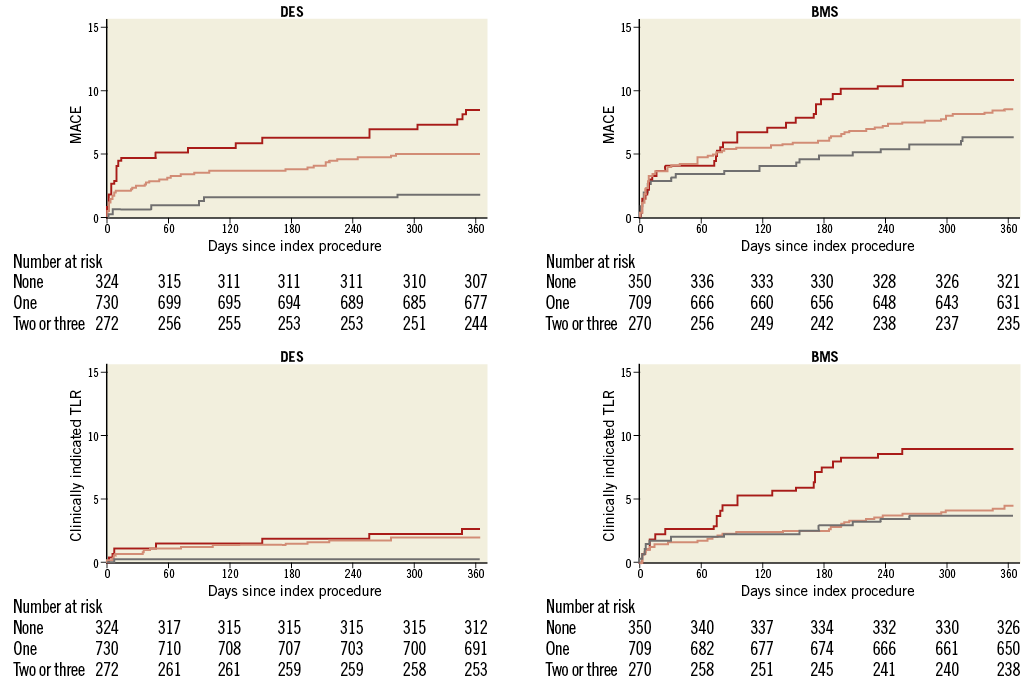
Figure 1. Major adverse cardiac events (MACE) and clinically driven target lesion revascularisation (TLR) at one year for the three risk score groups (0: grey, 1: pink, 2/3: red) and separately for drug-eluting stents (DES) and bare metal stents (BMS) used for primary angioplasty.
The rate of death was higher with a risk score of 1 or more; however, no difference was seen between DES and BMS in any of the groups.
The rate of reinfarction was higher with a risk score of one or more, but not different between DES and BMS in any of the groups.
The rate of clinically indicated target lesion revascularisation (TLR) was lower in patients with a score of 0. There was a significant reduction in TLR with DES in all three risk score groups. Patients without diabetes, large vessel diameters and short lesions (risk score 0) still derived a benefit from a lower TLR when treated with DES (0.3% vs. 3.7%, p=0.0161). Stent thrombosis was lower with the use of DES in all risk score groups. Table 4 gives details of clinical outcomes according to treatment and risk score.
A further analysis looking at the three risk criteria individually in relation to clinical endpoints is illustrated in Figure 2. Individual risk factors did not predict a differential benefit of DES with respect to a reduction of TLR. The reduction in clinically indicated TLR was significant across all patient groups, whether individual risk factors (diabetes, long lesion, small vessel) were present or not.
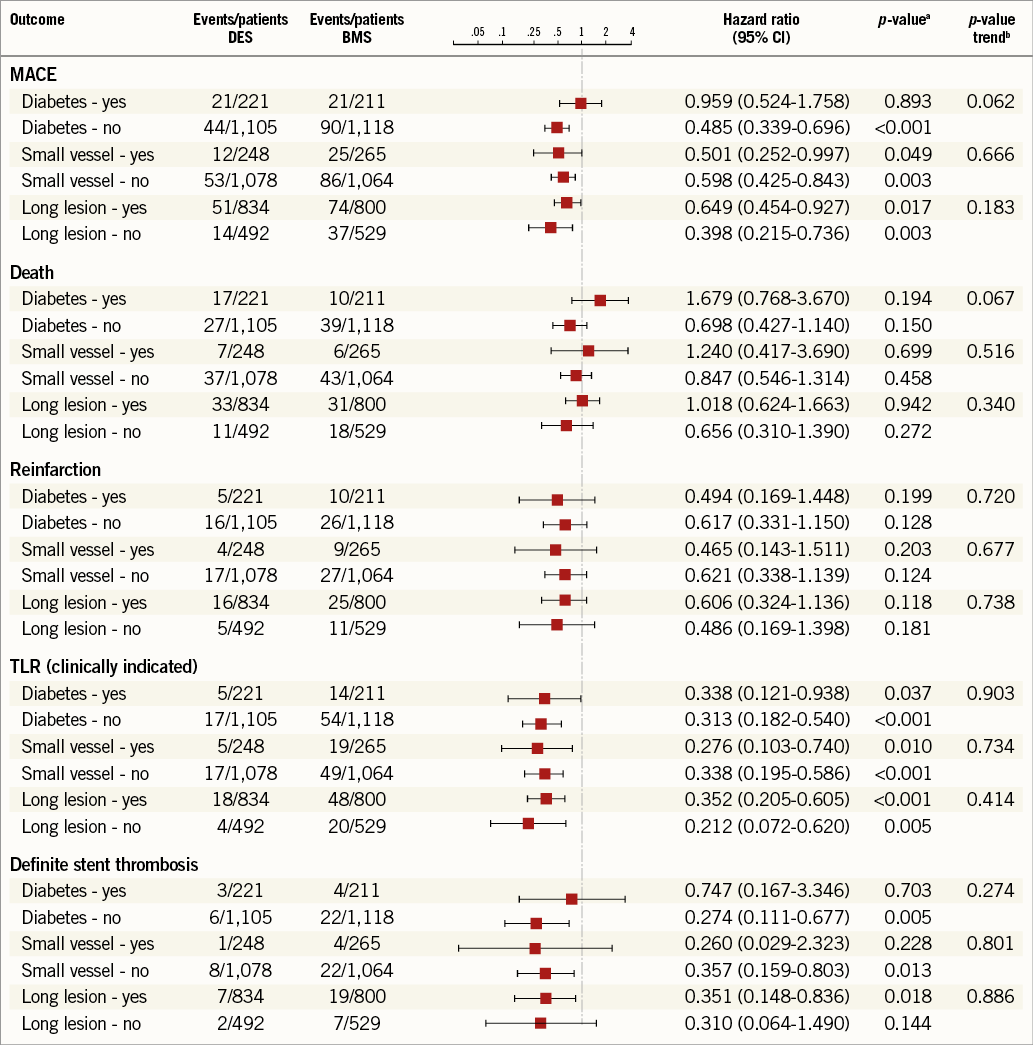
Figure 2. Individual pooled analyses of clinical outcomes in the presence of individual risk factors (lesion length, vessel size and diabetes) for DES vs. BMS use. ap-values random effects using separate Cox’s regressions per outcome and per presence or absence of the risk factor (stratified by study). bp-values for the interaction between stent type and presence/absence of risk factor from the full-factorial Cox’s regression (stratified by study).
The numbers needed to treat (NNT) in order to avoid one MACE with the use of DES were 21 in patients with a risk score of 0, 28 in patients with a risk score of 1, and 48 in patients with a risk score of 2 or 3.
Numbers needed-to-treat with a DES to avoid one TLR were 30 in patients with a risk score of 0, 41 in patients with a risk score of 1 and 16 in patients with a risk score of 2 or 3.
Discussion
The major finding of this pooled analysis is that, with the use of contemporary drug-eluting stents in primary PCI, a clinical benefit over bare metal stents was seen irrespective of the presence of risk criteria for restenosis. The use of bare metal stents in patients without anatomical or clinical risk factors (short lesions, large vessel and absence of diabetes) was still associated with a higher rate of repeat revascularisation.
The risk of adverse events, especially revascularisation, was higher in patients with diabetes, long lesions and small arteries. The number needed-to-treat to avoid one revascularisation with a DES vs. BMS was smallest when two or more risk factors were present. Contrary to the original hypothesis, the number needed-to-treat in patients without any of these criteria was still sufficient to justify the use of DES.
This is in contrast to published findings in patients receiving stents for the treatment of stable angina and a previous similar assessment in a propensity-matched analysis covering all percutaneous interventions in Ontario30. In this study with a similar risk score approach applied to the analysis, DES were associated with significant reductions in the rate of target vessel revascularisation among patients with two or three risk factors for restenosis (i.e., presence of diabetes, small vessels [<3 mm in diameter], and long lesions [>or=20 mm]) but not among lower-risk patients. Only a small proportion of these patients presented with STEMI.
NICE originally recommended the selective use of DES over BMS in lesion subsets with small target vessels and long target lesions, based on the analysis of early RCT for paclitaxel-eluting stents (TAXUS™; Boston Scientific, Marlborough, MA, USA) and sirolimus-eluting stents (CYPHER®; Cordis, Johnson & Johnson, Miami Lakes, FL, USA). The revised guidance from 2008, again a health economic analysis and recommendation, was based on a total of 17 RCT including second-generation DES. None of the trials included patients presenting with STEMI and these patients were excluded from the guidance.
Limited data are available in the context of primary PCI. In HORIZONS-AMI31, patients presenting with STEMI were randomised to receive either a PES or a BMS (3:1 randomisation). After three years, patients who received a PES had lower rates of ischaemia-driven TLR (9.4% vs. 15.1%; p<0.0001), with no significant differences in the rates of death, reinfarction, stroke or stent thrombosis22. A later analysis showed that insulin-treated diabetes mellitus (hazard ratio [HR]: 3.12), reference vessel diameter <3.0 mm (HR: 2.89), and lesion length ≥30 mm (HR: 2.49) were independent predictors of 12-month TLR after BMS10. In patients with two or three of these baseline risk factors, PES use markedly reduced 12-month TLR in comparison with BMS (19.8% vs. 8.1%, p=0.003). In patients with one of these risk factors, the 12-month rates of TLR were modestly reduced by PES (7.3% vs. 4.3%, p=0.02). The 12-month TLR rates were low and similar for both stents in patients with zero risk factors (3.3% vs. 3.2%, p=0.93).
In comparison with the presented studies, MACE rates in HORIZONS-AMI were in a similar range. The 12-month outcomes in the BMS arms were 3.5% for death in both COMFORTABLE AMI and HORIZONS-AMI, rates of reinfarction were 3.7% and 4.5%, and for ischaemia-driven TLR 5.7% and 7.4%, respectively.
A difference in outcome was noted in patients with no risk factors; the TLR rate in our pooled analysis demonstrated only one event (0.3%) in the DES group. This may reflect the advantage of a newer-generation DES in comparison with PES, and explains why in this study the benefit of DES was seen in the lowest risk patients as well.
Interestingly, in this cohort of patients undergoing primary PCI, none of the individual risk factors chosen for the score predicted an increased benefit of DES over BMS. The reduction in TLR was similar, whether diabetes, small vessel size or long lesion length was present or not.
The combination of more than one risk factor did increase the absolute risk of an event. However, the increase in the TLR rate was much less steep in the DES group than in the BMS group. In the highest risk group with two or more risk factors, the TLR rate with BMS was 8.9% compared to 2.6% in the DES group.
The NNT to avoid one MACE was higher in the higher-risk score group. This is a surprising finding as we would have expected a larger benefit in patients with more risk factors. This finding points towards a weakness of the chosen risk criteria to identify benefits of DES. The patient cohort with high risk scores may have had additional patient-related factors that reduced the effect of the TLR rate on the overall MACE.
When looking at cost-effectiveness, the price difference between DES and BMS has to be taken into account32. With clinical data suggesting a benefit in all subgroups of patients, selective rather than general use of contemporary DES for PPCI can only be justified in healthcare systems with a very large premium payable for the use of DES.
Limitations
The risk criteria of length and vessel diameter in this study are derived from the diameter and lengths of the stents used, rather than QCA analysis of the target lesion and vessel. The assessment of length and diameter may be more difficult in the setting of STEMI than in elective situations, and may lead to overestimation of length of the lesion and underestimation of true vessel size. The EXAMINATION trial included patients presenting >24 hours following symptom onset, and patients undergoing rescue angioplasty for failed thrombolysis. Hence, the studied population is heterogeneous and the effect of late presentation and increased infarct sizes may have diminished the clinical effect of DES over BMS. Neither trial included angiographic follow-up; consequently, the reported outcomes are clinically driven and may not reflect the angiographic incidence of in-stent restenosis. The two trials used DES with absorbable and permanent polymers. We cannot exclude different results with these individual devices.
Conclusions
In this large cohort of patients undergoing primary PCI for acute ST-elevation myocardial infarction, the use of drug-eluting stents showed a clinical benefit over bare metal stents in all patient groups. Contrary to common practice, selection of BMS for patients without risk factors (long lesions, small vessels, diabetes) would not translate into equivalent outcomes. The selective use of DES should be discouraged.
| Impact on daily practice The use of contemporary drug-eluting stents (DES) for primary percutaneous coronary intervention (PPCI) shows a clinical benefit over bare metal stents in all patients. Routine practice should adopt the universal use of contemporary DES. |
Guest Editor
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum München, Munich, Germany.
Acknowledgements
A Baumbach is supported by the Bristol NIHR Biomedical Research Unit.
Conflict of interest statement
A. Baumbach reports receiving speaker and advisory fees from Biosensors and Abbott Vascular and a research grant from Abbott Vascular. S. Windecker reports receiving research contracts to the institution from Abbott, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, and St. Jude Medical. S. Brugaletta reports receiving speaker fees from AstraZeneca, Abbott, BioVentrix and a research grant to the institution from AstraZeneca and Menarini. The other authors have no conflicts of interest to declare. The Guest Editor holds patents related to drug-eluting stent technology.
Supplementary data
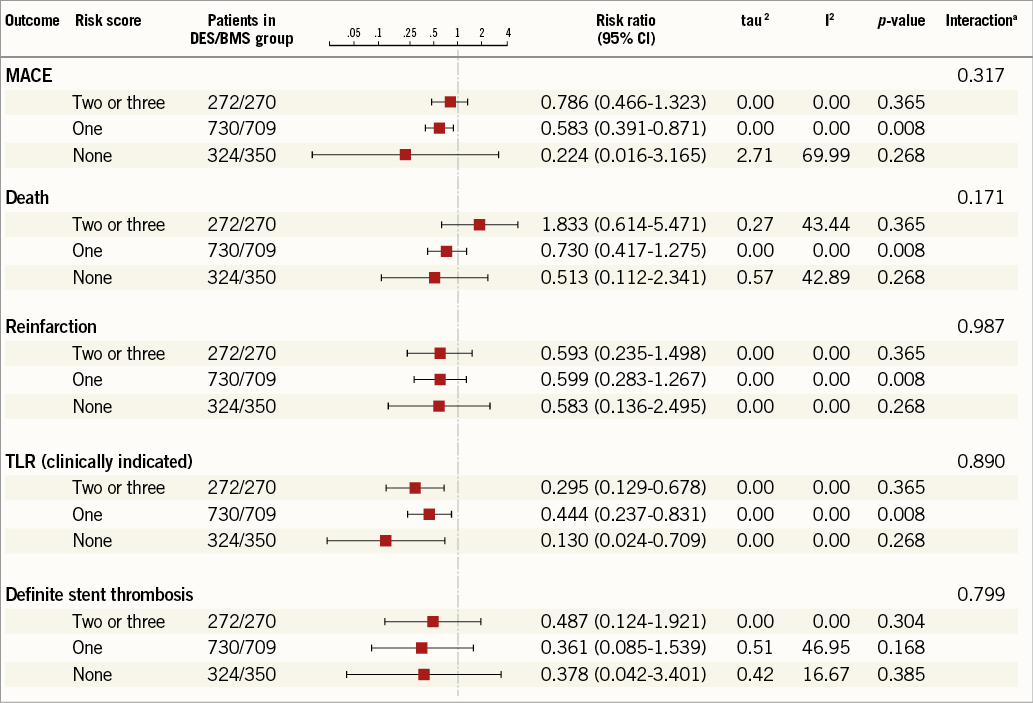
Online Figure 1. Pooled analysis of clinical endpoints according to stent used in the three risk score groups. p-values random effects using separate meta-analyses per outcome and risk score. ap-values from test for trend over NICE risk factors using separate meta-regressions per outcome.
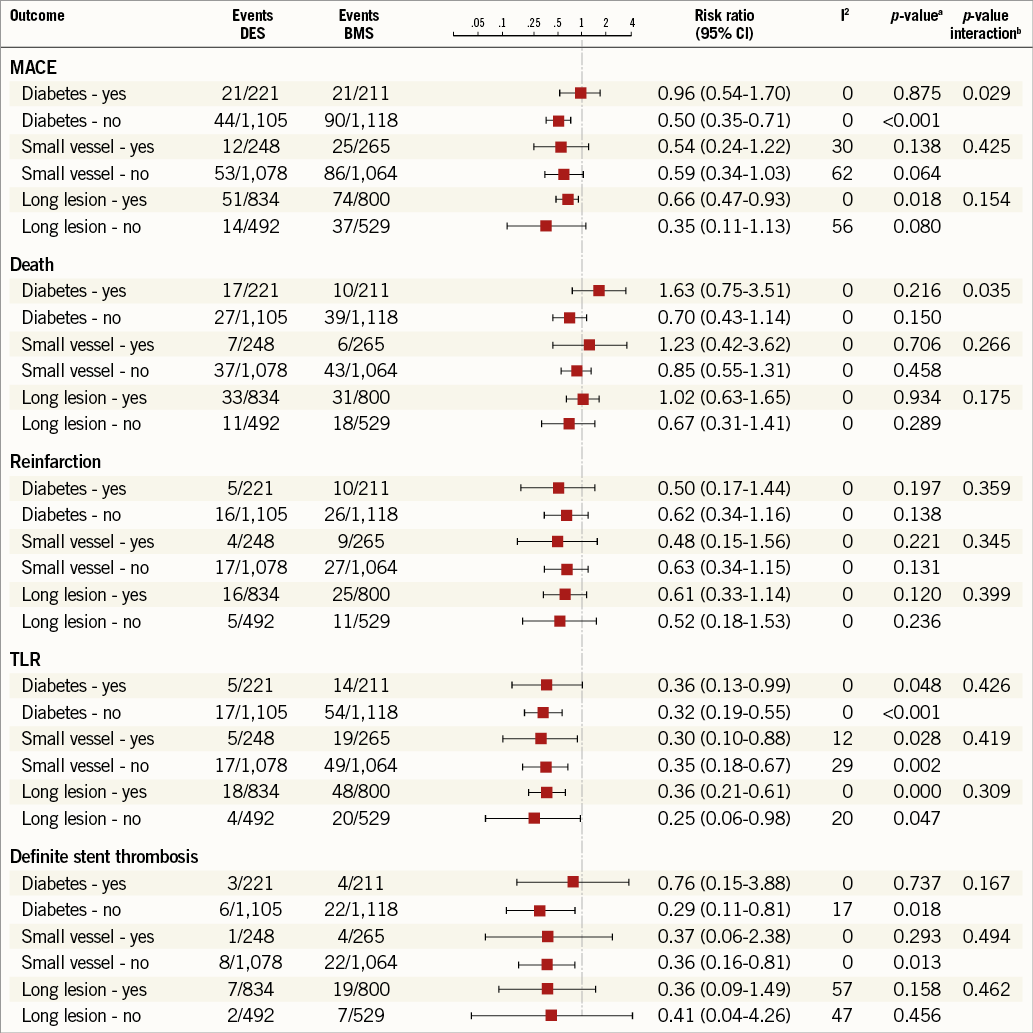
Online Figure 2. Meta-analysis of clinical outcomes in presence of individual risk factors (lesion length, vessel size and diabetes) for DES vs. BMS use. ap-values random effects using separate meta-analyses per outcome and per presence or absence of the risk factor; bp-values from a Z-test for the interaction between stent type and presence/absence of risk factor

