Abstract
Aims: Percutaneous edge-to-edge mitral valve repair with the MitraClip® was shown to be a safe and feasible alternative compared to conventional surgical mitral valve repair. We analyse the concept of the central clip and the predictors for the need of more than one MitraClip® in our high-risk surgical population with severe mitral regurgitation (MR).
Methods and results: Patients with severe MR (3 or 4+) and high operative risk (as defined by logistic EuroSCORE) refused for conventional mitral valve repair were considered for MitraClip®. The procedure was performed under general anaesthesia with transoesophageal echocardiographic (TOE) guidance. Device success was defined as placement of one or more MitraClips® with a reduction of MR to ≤2+. Patients were followed up clinically and with TOE at one month and one year. From September 2009 to March 2012, 43 patients with severe MR with a mean age of 74.8±10.7 years (30 males, 13 females; mean logistic EuroSCORE 24.1±11, mean LVEF 47.5±18.5%; mean±SD) were treated. Median follow-up was 385 days (104-630; Q1-Q3). Device implantation success was 93%. All patients were treated following the central clip concept: 52.5% of MR was degenerative in aetiology and 47.5% was functional. The degree of MR was reduced from 3.6±0.4 to 1.4±0.6 (p<0.001); NYHA Class improved from 3.1±0.4 to 1.8±0.7 (p<0.001). Nineteen patients (47.5%) received two or more clips. Vena contracta (p<0.001) and the presence of two broad jets (p<0.001) were correlated with the need for a second clip. The presence of a restricted posterior mitral valve leaflet (PML) was inversely correlated with the need for more than one clip (p=0.02). A cut-off value of ≥7.5 mm for vena contracta predicted the need for a second clip (sensitivity 83%, specificity 90%, p=0.01).
Conclusions: The central MitraClip® concept achieved a significant reduction in the degree of mitral regurgitation in the majority of patients treated. The presence of a broad jet (quantified by a vena contracta greater than 7.5 mm) significantly predicted the need for more than one clip.
Introduction
Conventional surgical repair or replacement has been the standard of care for symptomatic severe mitral regurgitation (MR)1-3. However, before the emergence of transcatheter valve therapies, optimal medical therapy and cardiac resynchronisation therapy in selected candidates were the only treatment for patients deemed too high risk for conventional surgery4-7. Percutaneous edge-to-edge mitral valve repair with the MitraClip® system (Abbott Laboratories, Abbott Park, IL, USA) was demonstrated to be a safe and feasible alternative to surgical treatment for severe MR, though less effective at reducing MR than conventional surgery8-11. Adverse valve morphology and severe left ventricular dysfunction have been the two major challenges in treatment with the MitraClip® system12-14. We report our experience in MitraClip® for patients with severe symptomatic MR who are high risk for conventional surgery, focusing on the determinants of the need for more than one clip.
Methods
PATIENT SELECTION AND PREOPERATIVE ASSESSMENT
Forty-three consecutive patients who underwent elective edge-to-edge MitraClip® implantation for symptomatic severe chronic MR (grade 3+ or 4+) with high operative risk were prospectively collected within the hospital database. Surgical risk assessment was based on either the EuroSCORE or the presence of specific surgical risk factors not covered by the EuroSCORE. The decision to undergo MitraClip® implantation was based on a multidisciplinary assessment of the patient´s surgical risk and anatomical features of the valve. The exclusion criteria used were quite different from the commonly applied EVEREST criteria15. In particular, partially calcified leaflets, length and depth of coaptation, width and maximal gap of the flailed leaflet, absolute valve area <4.0 cm2, poor ejection fraction, and location of jet origin were not absolute contraindications12,16. Patients were considered unsuitable only when there was reasonable concern that the MitraClip® would fail to grasp both leaflets or would induce a critical reduction of mitral valve area. Active endocarditis and poor life expectancy were the only absolute exclusion criteria. All patients underwent preoperative transthoracic and transoesophageal echocardiography (TOE) prior to intervention to assess the mitral valve morphology, severity of mitral regurgitation and anatomical suitability for MitraClip® implantation17,18. Clinical assessment included identifying symptoms and New York Heart Association (NYHA) functional Class, and a lung function test in those with a history of pulmonary disease.
PROCEDURAL DETAILS
The procedure was performed under general anaesthesia in a catheterisation laboratory in our institution. Both fluoroscopic guidance and three-dimensional transoesophageal echocardiographic (3D-TOE) guidance were utilised. The right femoral vein was used as the primary vascular access with concomitant placement of a 5 Fr arterial sheath in the contralateral femoral artery to monitor the arterial blood pressure during the procedure in the initial cases. A 24 Fr sheath was placed in the right femoral vein with transseptal puncture performed under transoesophageal real-time guidance as previously described3. After intravenous heparin administration to achieve an activated clotting time of >250 seconds, a 22 Fr steerable sheath was advanced 1-2 cm in the left atrium. The MitraClip® device is mounted on a steerable catheter allowing correction of the medial/lateral and anteroposterior orientation in order to navigate safely within the left atrium under TOE guidance. We performed all MitraClip® implantations following the central clip concept, meaning that we initially positioned the clip in the centre of the jet, splitting it into two symmetrical jets (Figure 1). When the clip was partially closed, we confirmed the effective MR reduction into two small jets. If the two resultant jets were broad and significant, we decided to reposition the first clip more medially, switching to a two-clip strategy. After deploying the first clip medially, we implanted a second clip more laterally, confirming the absence of significant MV gradient before release. However, when the jet origin was very broad, we considered two clips from the beginning. Once the device was positioned centrally in the mitral valve orifice, the two arms of the MitraClip® were opened and oriented perpendicular to the mitral closure line using 3D-TOE (Figure 2). The MitraClip® was then retracted to capture the two leaflets aiming at the source of maximal regurgitation. Reduction of MR was assessed immediately by TOE and on day four postoperatively by transthoracic echocardiography (TTE). When facing major difficulties grasping both leaflets, controlled asystole was induced as described elsewhere19.
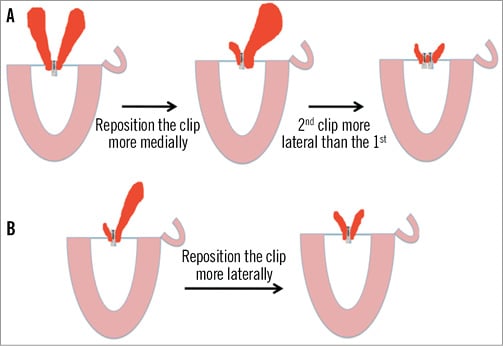
Figure 1. The “central clip” concept. MitraClip® implantation changes in strategy depending on the behaviour of the MR after positioning the first clip in a central position. If the jet origin is very broad in a P2 prolapse, a two-clip strategy should be considered from the beginning of the procedure.
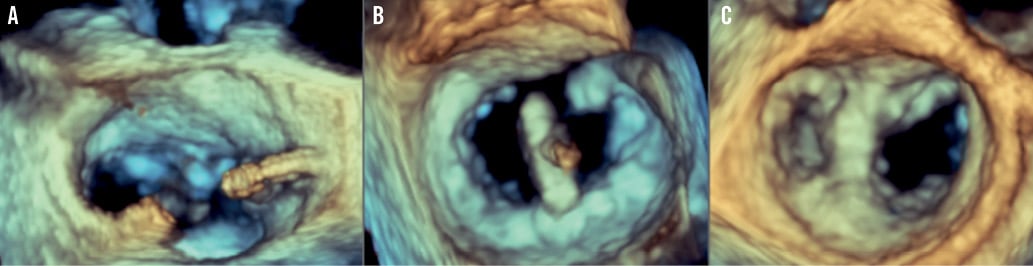
Figure 2. 3-D transoesophageal echocardiographic (3D-TOE) guidance during MitraClip® implantation. A) After transseptal puncture, the position and distance to the mitral valve are measured. A high and posterior puncture is recommended . B) Orientation of the clip in the mitral valve, perpendicular to the MV opening, assessed by 3D-TOE, from the left atrium (LA). C) Final result after MitraClip® implantation from the LA with two MV orifices, mimicking the Alfieri surgical technique.
The patients were extubated on the same day after the procedure in the recovery room where the haemodynamic status was monitored overnight. They would be transferred to the step-down high dependency unit the next morning and then to the cardiology ward where they would start to mobilise and receive further medication titration.
MEDICATION AND FOLLOW-UP
Anticoagulation or antiplatelet therapy was individualised based on the presence of atrial fibrillation, concomitant coronary artery disease, previous coronary stent implantation and bleeding risk of patients. All patients received aspirin 75 mg daily for at least three months and clopidogrel for four weeks unless they were receiving warfarin, in which case warfarin would be continued post-procedurally with INR targeted at 2-3.
Echocardiographic assessments pre-procedurally and post-procedurally or at follow-up were based on American Society of Echocardiography guidelines20. In particular, the severity of MR was assessed according to the technique previously described21-23. Clinical and echocardiographic follow-up were scheduled at four weeks, three months, six months and one year thereafter.
OUTCOME MEASURES
Procedural success was defined as successful and stable MitraClip® placement with residual MR ≤2+ upon discharge. Major adverse events at 12 months were defined as a composite of cardiovascular mortality, myocardial infarction, unplanned cardiac surgery, transfusion of more than two units and heart failure requiring hospitalisation. Clinical assessment and echocardiography were carried out at predefined periods as mentioned above.
Statistics
Continuous variables are expressed as mean±SD when normally distributed and as medians with interquartile ranges when not normally distributed. Paired Student’s t-tests or Mann-Whitney U tests were utilised to assess the differences in the means of continuous variables before and after the procedures.
The relationship of various parameters with the implantation of two MitraClip® devices was investigated with Spearman’s rank-order correlation coefficient. The variables identified as having significant univariate correlations were entered in a multivariate binary logistic regression model after ensuring that the assumptions regarding sample size, multicollinearity and presence of outliers were not violated. ROC curves were plotted for the parameters identified as independent determinants of the utilisation of two clips, in the multivariate model. In our study population, the best cut-off values for the prediction of the implantation of two MitraClips® were determined, aiming at a maximum sum of sensitivity and specificity. A p-value of <0.05 was considered statistically significant for all tests. Analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Forty-three consecutive patients with a mean age of 74.8±10.7 years were included in the study (30 males and 13 females). Among the 43 patients, the MitraClip® was successfully implanted in 40 patients (overall procedural success of 93%) (Figure 3). Table 1, Table 2 and Table 3 summarise the baseline characteristics, pre-procedural and post-procedural features of the population. Twenty-one patients received one clip (group 1) and 19 received more than one clip (group 2). Eighteen patients were treated with two clips and one patient required three clips (Figure 4). Twelve patients (52%) had functional MR in group one and seven (37%) in group two. Eight patients (20%) had LVEF of ≤25%: seven of them belonged to group one where only one clip was implanted. Mean logistic EuroSCORE was 24.1±11, 23±8.7 in group one and 25.3±14.4 in group 2 (p=0.56). Left ventricular (LV) ejection fraction was 47.5±18.5%, 44.6±21.1% in group one and 50.9±14.7% in group two (p=0.29).
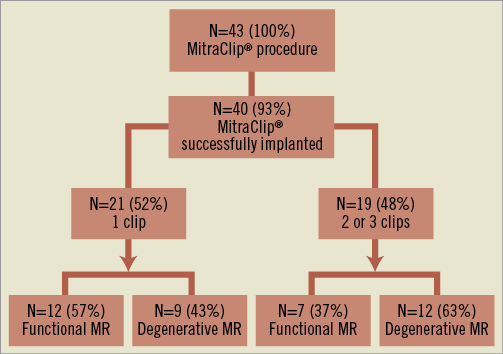
Figure 3. Enrolment of the patients.
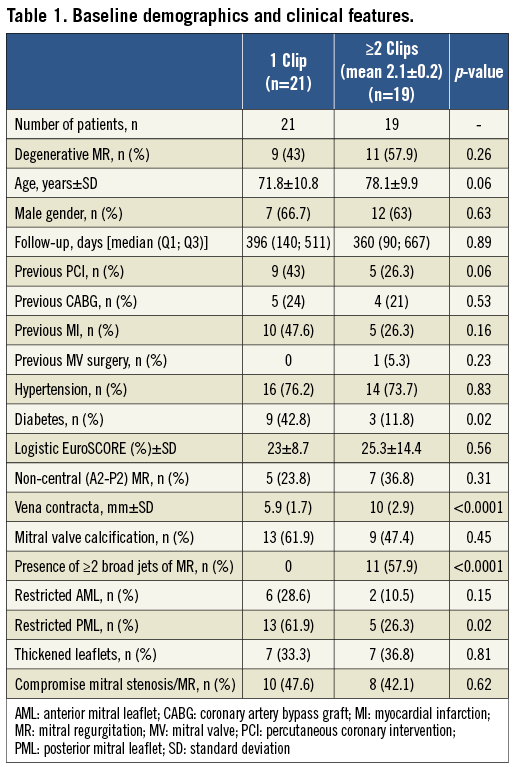
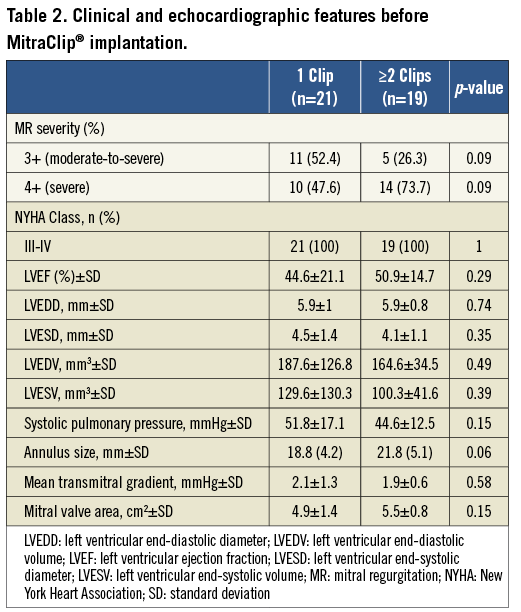
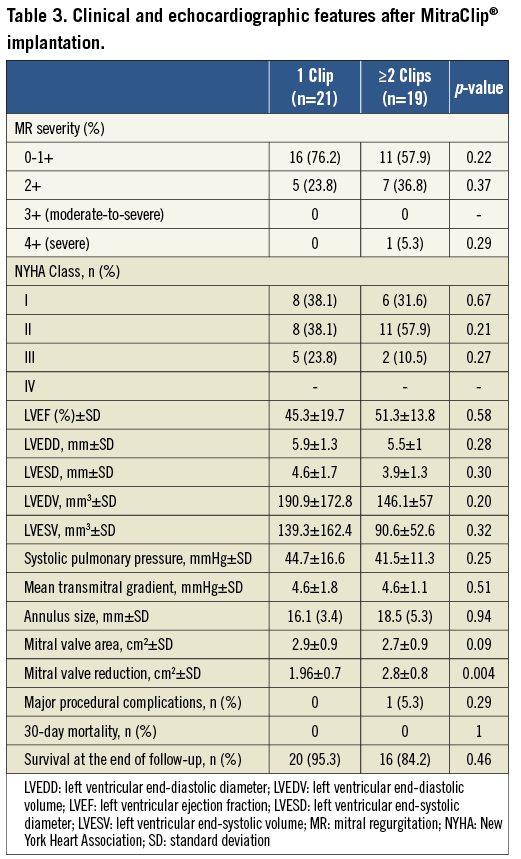
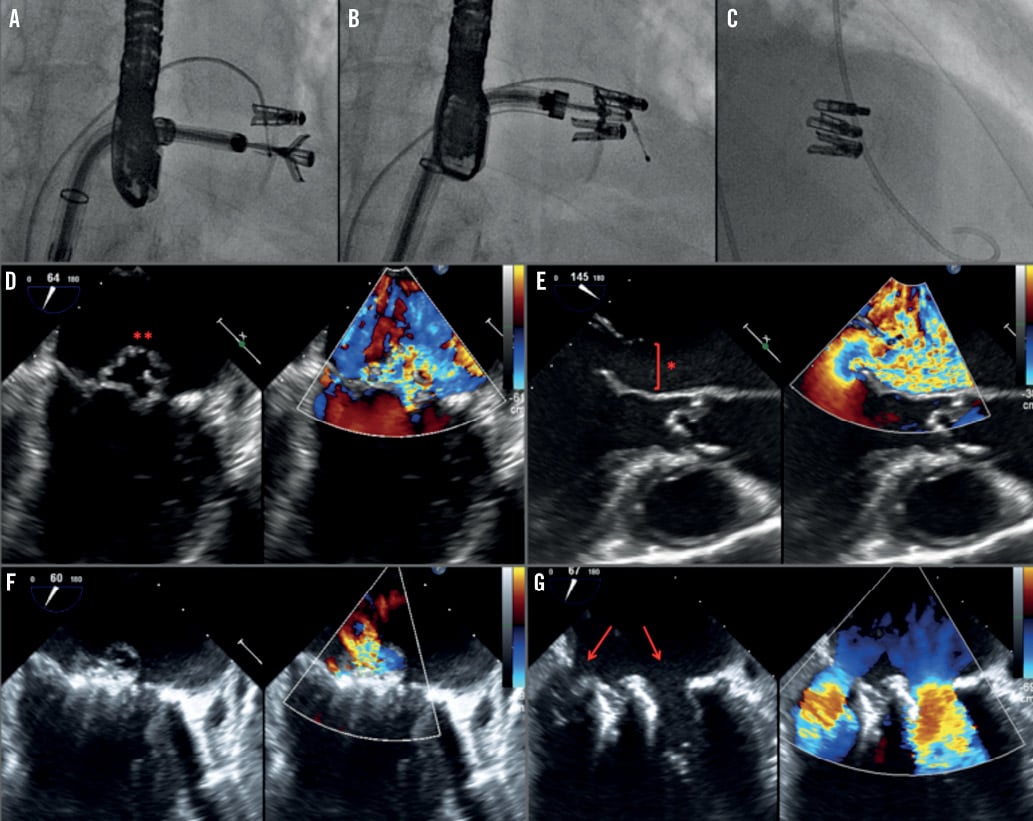
Figure 4. An 82-year-old hypertensive male with severe MR caused by MV prolapse (A-G). After temporary pacemaker insertion, transseptal puncture was performed and the catheter delivery system inserted (A-C). A thickened posterior mitral leaflet prolapsed into the left atrium (D-E, red asterisks). Due to the broad jet of the mitral regurgitation (vena contracta of 17 mm), a strategy with various clips was planned. After deployment of the first clip (A), a second and a third clip were needed to reduce the severity of the MR. TOE confirmed a reduction of the MR severity from 4+ to 1+ after the third clip (F-G), with a reduction of the MV area from 4.6 cm2 to 1.8 cm2.
In patients receiving one clip (group one), all patients (100%) had a reduction of MR to ≤2+ at the end of follow-up, compared to 94.7% of the patients receiving two clips (p=0.38) (Figure 5). NYHA Class improved in both groups after MitraClip® implantation, with more than 82% of the patients being ≤Class II at the end of follow-up. Median follow-up was 396 days (140; 511) in group one and 360 days (90; 667) in group two (p=0.89), without in-patient or 30-day mortality. Major adverse events occurred in five patients (12%), with successful MitraClip® implantation. One patient in group one died at 297 days after the procedure due to further deterioration in left ventricular function presenting with heart failure. In group two, three patients died during the follow-up (at 75, 90 and 360 days) due to further deterioration in left ventricular function and heart failure, with a mean age of 83 years. One patient in group two with previous mitral valve repair underwent surgical bail-out MV repair after the MitraClip® was unable to reduce the degree of MR. Surgeons successfully repaired the valve and removed the two clips implanted, documenting the presence of a MV cleft responsible for the MR, undetected by the preoperative TOE. In comparisons between the functional MR and degenerative MR subgroups, clinical outcomes in terms of reduction of MR and improvement in NYHA functional class did not show any significant difference (Table 2 and Table 3). Details of the results and comparisons between one clip and ≥2 clips are shown in Table 1, Table 2 and Table 3.
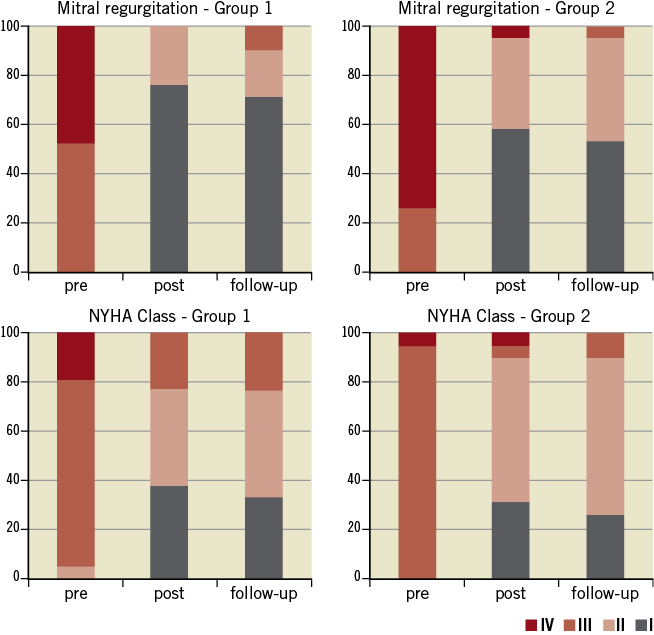
Figure 5. Mitral regurgitation (MR) reduction and NYHA Class improvement after MitraClip® implantation. Upper panels show the reduction of MR achieved after MitraClip® implantation in both groups. Inferior panels show the improvement in NYHA functional Class after MitraClip® implantation.
In our series, there was a predominance of patients with degenerative MR receiving MitraClip® implantation, different from other reported series in Europe. Despite the fact that in group two patients received more than one clip, the compromise between the reduction of MR and the creation of mitral stenosis (MS) was similar in both groups (47.6 % in group one vs. 42.1% in group two, p=0.62). Mitral valve area reduction was significantly greater in patients receiving ≥2 clips compared to those receiving one clip (2.8±0.8 cm2 vs. 1.96±0.7 cm2, p=0.004). However, comparing group one and group two, the mean gradient across the mitral valve showed no differences pre-implantation (2.1±1.3 mmHg vs. 1.9±0.6 mmHg, respectively, p=0.58) or post-implantation (4.6±1.8 mmHg vs. 4.6±1.1 mmHg, respectively, p=0.51). Of note, a mean gradient ≥5 mmHg was often taken as a relative contraindication for further clip implantation.
We found a similar proportion of mitral valve (MV) calcification (62% vs. 47%, respectively, p=0.45) and thickened leaflets (33% vs. 37%, p=0.81) in both groups. However, patients receiving one clip had a higher prevalence of restrictive posterior mitral valve leaflet (PML) compared to those receiving more than one clip (62% vs. 26%, p=0.02). In our series, 23.8% and 36.8% of the patients had non-central pathology (different from A2/P2) in groups one and two, respectively. Moreover, the presence of a broad regurgitation jet measured by the vena contracta was significantly higher in group two compared to group one (10±2.9 mm vs. 5.9±1.7 mm, p<0.001). In addition, the presence of two broad separated jets of regurgitation was highly prevalent in group two compared to group one (58% vs. 0%, p<0.001). In the multivariate analysis, the vena contracta was the only independent predictor of the need for a second clip (OR 2.5, 95% CI: 1.2-5.3, p=0.013). A cut-off value of ≥7.5 mm had high sensitivity and specificity (83% and 90%, respectively) as an independent predictor of a second clip implantation (Figure 6).
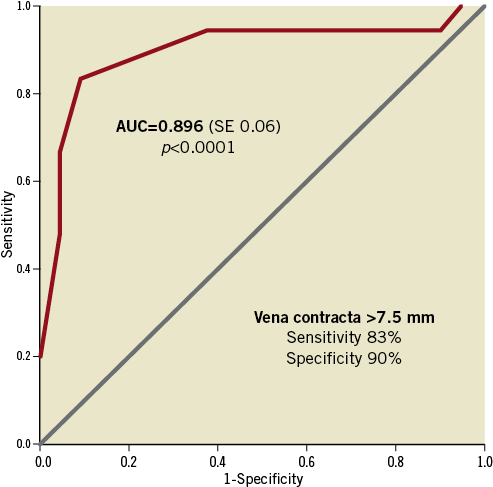
Figure 6. Predictive value of the vena contracta for the need of more than 1 clip. ROC curve represents the accuracy of the vena contracta measurement as a predictor of the need for more than 1 MitraClip® in our population. The area-under-the-curve (AUC) was 0.896 for a cut-off point of 7.5 mm.
Discussion
Our experience and the characteristics of the two groups are presented. Our data strongly support the central clip concept (Figure 1), since patients in group two had significantly broader jets and almost 60% of patients in group two had two broad jets. We used a third clip in a patient with massive MR with a very broad jet (Figure 4). On multivariate analysis, the presence of a broad jet measured using the vena contracta significantly predicted the need for a second clip. Using a ROC analysis, the cut-off point of 7.5 mm was predictive of the need for a second clip (83% sensitivity, 90% specificity) (Figure 6). The presence of a restricted PML was present in 62% of our one-clip group. This feature does not prohibit the implantation of a second clip, but it may increase the risk of creating a significant MV gradient. MVA reduction in the group with one clip compared to the group with more than one clip was 1.96±0.7 vs. 2.8±0.8 cm2, p=0.004, respectively, indicating an effective MVA reduction in both groups, significantly greater in the group of patients receiving more than one clip. However, the compromise between MR and MS was balanced in both groups. Of note, group two had larger baseline MVA and this feature could impact on the reduction observed after the procedure.
Our study population expands the results of the EVEREST trial11 with an overall 51% of patients presenting with functional MR. The prevalence of degenerative MR was greater in the two-clip group (58%), as expected. At one-year median follow-up, reduction of MR to ≤2+ was achieved in 93% of patients, which is comparable to the results in the published series1,2,11,15,23. MitraClip® was shown to be a safe procedure in our study as there was only one patient referred to MV surgery 24 hours after MitraClip® implantation due to persistent MR 3-4+. No other major adverse events periprocedurally nor 30-day mortality were documented. Four patients had died at the end of follow-up because of heart failure related to dilated cardiomyopathy as all patients had functional MR and significant LV dysfunction at baseline. For those patients with successful MitraClip® implantation, clinical benefits were demonstrated in terms of reduction of MR severity, improvement in NYHA Class and improvement in LV size. In our study, more than 75% of the patients were in NYHA Class I-II at the end of follow-up in both groups. The reduction in LV diameters and volumes after MitraClip® implantation showed a non-significant trend towards greater reduction in the two-clip group. This is possibly explained by the greater percentage of patients with functional MR in the two-clip group and because few patients experienced progressive worsening of LV systolic function in the presence of dilated cardiomyopathy despite successful reduction of MR by MitraClip®.
In our study, 93% of patients were able to achieve a reduction of MR to ≤2+ which was a promising result. In this technically demanding procedure, co-operation and communication between the operators and the person performing the TOE are of great importance, not only during the positioning of the clips and the assessing of MR reduction but also in determining the site of optimal transseptal punctures.
Despite the absence of complete reduction of MR in both groups, together with the lack of significant improvement in LV ejection fraction and reduction in pulmonary arterial pressure, significant improvement in symptoms, in terms of reduction in NYHA Class, was observed in patients with a successful procedure. This phenomenon was also observed in other similar studies1,11,23. Such an improvement was also demonstrated in previous studies including the landmark EVEREST II study15. Comparing the one and two-clip sub-groups, there was no significant difference in terms of degree of improvement despite the difference in baseline LV function between the two groups (44.6±21.1 vs. 50.9±14.7 %, p=0.29, respectively). This showed that a reduction of MR compared with baseline would lead to a meaningful symptomatic improvement as well as a perceived improvement in the general health of these patients who often had multiple comorbidities.
Of note, during the implantation of MitraClip®, there is very often a compromise between complete reduction of MR and resultant mitral stenosis by placing further clips. In these patients, significant improvement in symptoms could still be achieved despite incomplete reduction of MR. This is evidenced by the fact that, despite MR severity being 1.6±0.6 at 12 months after MitraClip® therapy, patients still perceived improvements in symptoms.
Conclusions
MitraClip® was shown to be an effective and safe treatment for patients with severe functional and degenerative MR. The need for a second clip is determined by a broad jet of regurgitation (qualified by a vena contracta greater than 7.5 mm) or the presence of two broad jets. A restricted posterior mitral leaflet was a limitation to the use of more than one clip.
Study limitations
Owing to the relatively small number of patients recruited, further studies evaluating MitraClip® therapy are needed to draw a more meaningful conclusion, clarifying which patients might benefit from more than one clip using the central clip concept.
Acknowledgements
This project was supported by the NIHR Cardiovascular Biomedical Research Unit of Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Conflict of interest statement
The authors have no conflicts of interest to declare.

