
Abstract
Aims: We aimed to evaluate the impact of the complexity of coronary disease as assessed by the SYNTAX score (SXscore) on the clinical outcomes in the AIDA trial.
Methods and results: In the AIDA trial, we compared Absorb versus XIENCE in routine clinical practice. Clinical outcomes were stratified by SXscore tertiles: SXlow (SXscore ≤8), SXmid (SXscore >8 and ≤15) and SXhigh (>15). The SXscore was available in 1,661 of the 1,845 (90%) patients. The event rate of TVF was numerically lower in Absorb compared to XIENCE (3.7% versus 5.6%; p=0.257) in the SXlow tertile, numerically higher in Absorb in the SXmid tertile (11.4% versus 9.3%, p=0.421) and similar in the SXhigh tertile (15.5% versus 15.6%; p=0.960). The rates of definite/probable device thrombosis in Absorb versus XIENCE were significantly higher in the SXmid tertile (3.3% versus 0.8%, p=0.043) and in the SXhigh tertile (3.7% versus 0.8%, p=0.006).
Conclusions: We found no significantly different rates of TVF between Absorb and XIENCE patients. Absorb-treated patients in the SXmid and SXhigh tertiles had an increased risk of device thrombosis when compared to XIENCE-treated patients. The rates of device thrombosis in the SXlow tertile, while still higher for Absorb, are more acceptable than in the SXmid and SXhigh score tertiles.
Introduction
Despite encouraging initial short-term and long-term safety and efficacy in the ABSORB studies1,2,3, increased scaffold thrombosis rates in randomised controlled trials and in registries have been reported4,5,6. The Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial (AIDA trial) confirmed these concerns about the increased risk of scaffold thrombosis (ScT)7. However, its primary endpoint of non-inferiority on target vessel failure (TVF) at two-year follow-up was met8. The extent of coronary artery disease may affect outcomes after percutaneous coronary intervention (PCI). The SYNTAX score (SXscore) is an angiographic score of the coronary anatomy and lesion characteristics, which can be used as a measure of coronary artery disease complexity9. The SXscore has prognostic value in patients with de novo coronary artery disease undergoing revascularisation, and is associated with the burden of atherosclerotic plaque(s)10,11. Furthermore, it has been used to compare clinical outcomes after PCI with first- and second-generation drug-eluting stents (DES) in a variety of clinical and interventional settings12,13,14. In this pre-specified subgroup analysis of the AIDA trial, we evaluated the impact of SXscore on clinical outcomes.
Methods
The detailed study outline, the preliminary results, and the full two-year results of the AIDA trial were published previously7,8,15. SXscore was assessed using baseline diagnostic angiograms. All lesions were combined to provide the overall SXscore. Each coronary lesion with a diameter stenosis ≥50% in vessels ≥1.5 mm was scored. SXscore calculations were performed by core laboratory analysts blinded for clinical events (Cardialysis B.V., Rotterdam, the Netherlands). Occluded infarct-related arteries were scored as occlusions of unknown duration in a similar manner to any chronically occluded artery. Patients with in-stent restenosis lesions (non-target lesions) were scored in the same manner as a de novo lesion (www.syntaxscore.com).
STUDY ENDPOINTS
The primary endpoint of this substudy was TVF, defined as a composite of cardiac death, target vessel-related myocardial infarction (TV-MI) and target vessel revascularisation (TVR).
Secondary endpoints were TLF (a composite of cardiac death, TV-MI and target lesion revascularisation [TLR]), any revascularisation, all death, all myocardial infarction and device thrombosis. An independent clinical events committee (Cardialysis B.V., Rotterdam, the Netherlands) assessed all clinical endpoints according to the definitions of the Academic Research Consortium or the third universal definition of myocardial infarction.
STATISTICAL ANALYSIS
A pre-specified subgroup analysis, stratified by the tertiles of the SXscore, of clinical outcomes was performed. Analyses were performed according to the intention-to-treat principle. Event rates were based on Kaplan-Meier estimates, and Kaplan-Meier curves were compared by means of the log-rank test. Cox regressions were used to determine hazard ratios with 95% confidence intervals (CI) to compare the outcomes between the stent types across SXscore tertiles. Normally distributed continuous variables were presented as the mean with standard deviation (SD), variables with a skewed distribution as the median with interquartile range (IQR). These variables were compared with the ANOVA and the Kruskal-Wallis test, respectively. Categorical variables were presented as numbers and percentages and were compared with the chi-square test for trends. All statistical analyses were performed using SPSS software, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
SYNTAX SCORE AND BASELINE CHARACTERISTICS
In the overall AIDA study population, 924 patients were randomised to Absorb™ and 921 patients were randomised to XIENCE (both Abbott Vascular, Santa Clara, CA, USA). The SXscore was prospectively calculated in 1,661 of the 1,845 patients (90%). The predominant reason for not being able to calculate the SXscore was the unavailability of baseline angiograms with visualisation of both coronary arteries. The SXscore ranged from 1 to 57, with a mean±SD of 12.9±8.5. The SXscore tertiles were defined as SXlow (SXscore ≤8) (n=589), SXmid (SXscore >8 and ≤15) (n=538), and SXhigh (>15) (n=534). Full patient characteristics, target lesion characteristics, and procedural characteristics according to the three SXscore tertiles are summarised in Table 1, Supplementary Table 1 and Supplementary Table 2.
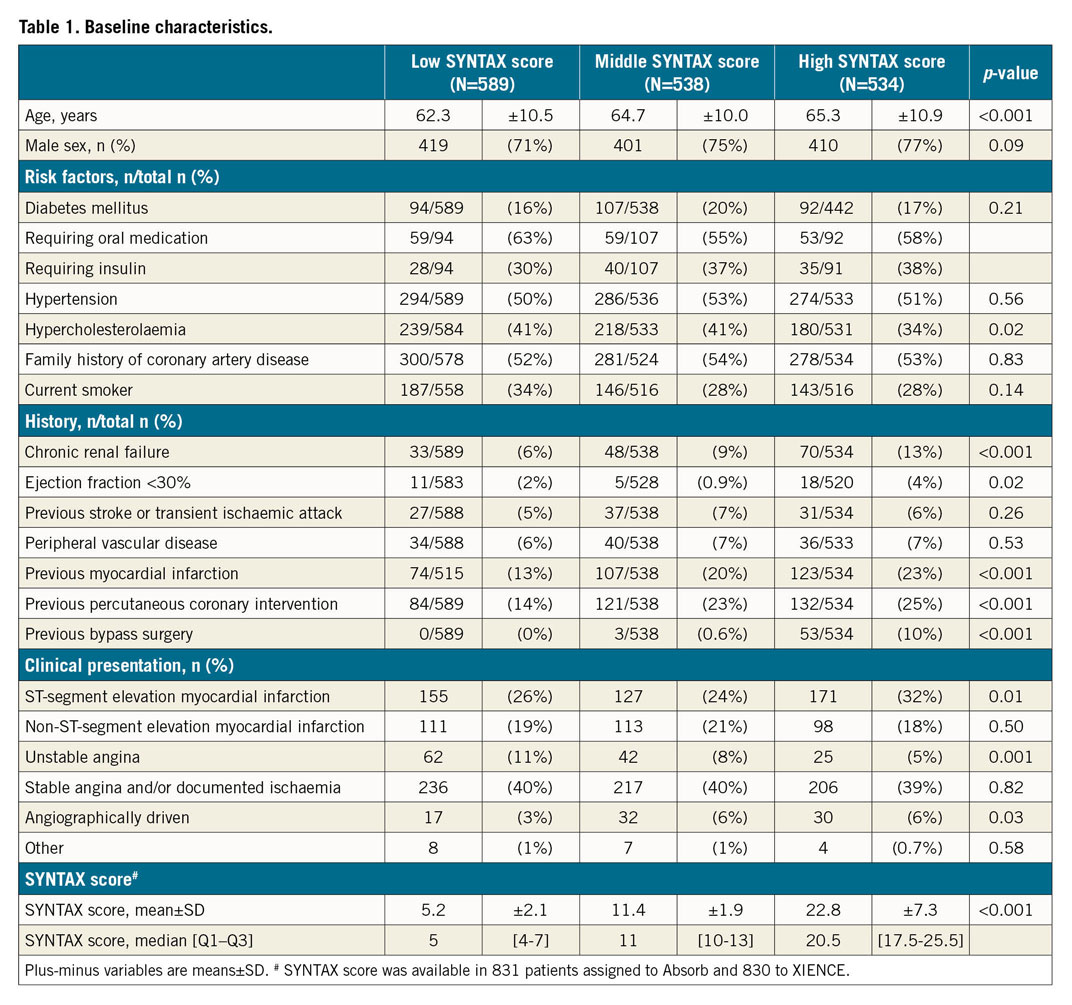
OVERALL CLINICAL OUTCOMES
At two-year follow-up, the Kaplan-Meier estimates of the TVF rate for the overall AIDA study population were 15.5% in the SXhigh tertile, 10.4% in the SXmid tertile and 4.7% in the SXlow tertile (p<0.001). The occurrence of definite or probable device thrombosis was significantly higher in the SXhigh tertile (SXhigh 2.9% versus SXmid 2.5% versus SXlow 0.7%; p=0.023) (Table 2).
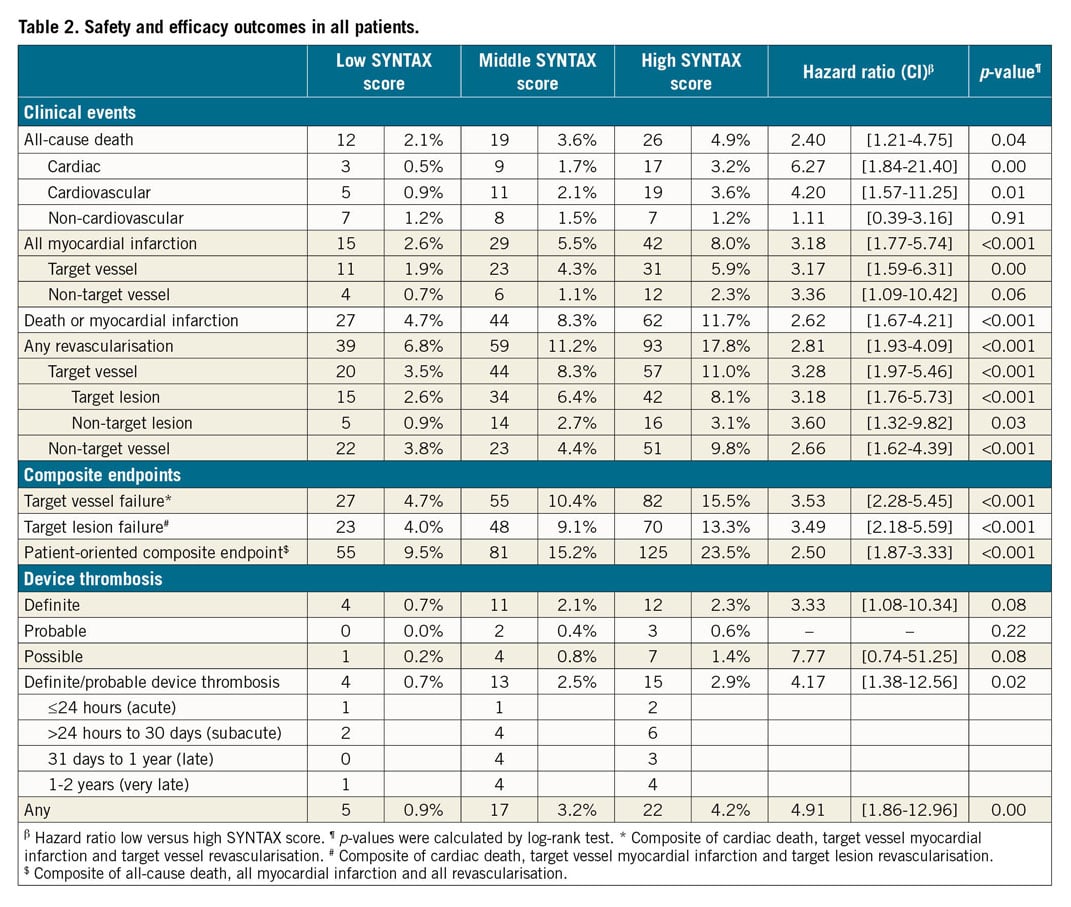
ABSORB CLINICAL OUTCOMES
Within the Absorb group, Kaplan-Meier estimates for TVF were significantly higher in the SXhigh tertile (15.5%; p<0.001) and in the SXmid tertile (11.4%; p=0.001) as compared with the SXlow tertile (3.7%). No statistically significant difference was observed between the SXmid and SXhigh tertiles (p=0.160) (Figure 1). Cardiac death rates did not differ significantly among the three tertiles (Figure 2A). Rates of TV-MI were significantly higher in the SXhigh tertile (7.4%; p=0.004) and in the SXmid tertile (5.5%; p=0.016), as compared to the SXlow tertile (1.5%). No difference was observed between the SXmid and SXhigh tertiles (p=0.366) (Figure 2B). The same observation was found for the rates of TVR and TLR, with statistically significant higher revascularisation rates for the SXhigh and SXmid tertiles as compared to the SXlow tertile (Figure 2C, Figure 2D). The rates of definite ScT were numerically, but not statistically significantly higher for the SXhigh tertile (3.7%; p=0.060) and the SXmid tertile (3.3%; p=0.094) versus the SXlow tertile (1.1%).
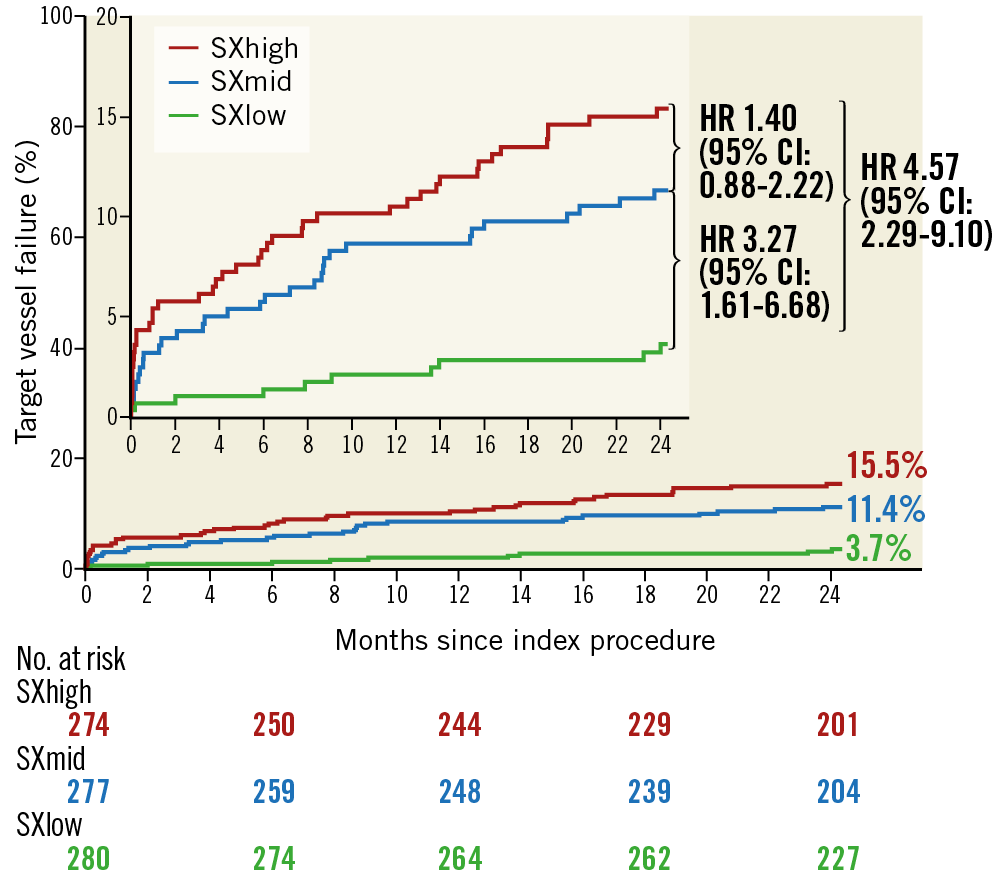
Figure 1. Kaplan-Meier curves for target vessel failure within the Absorb arm.
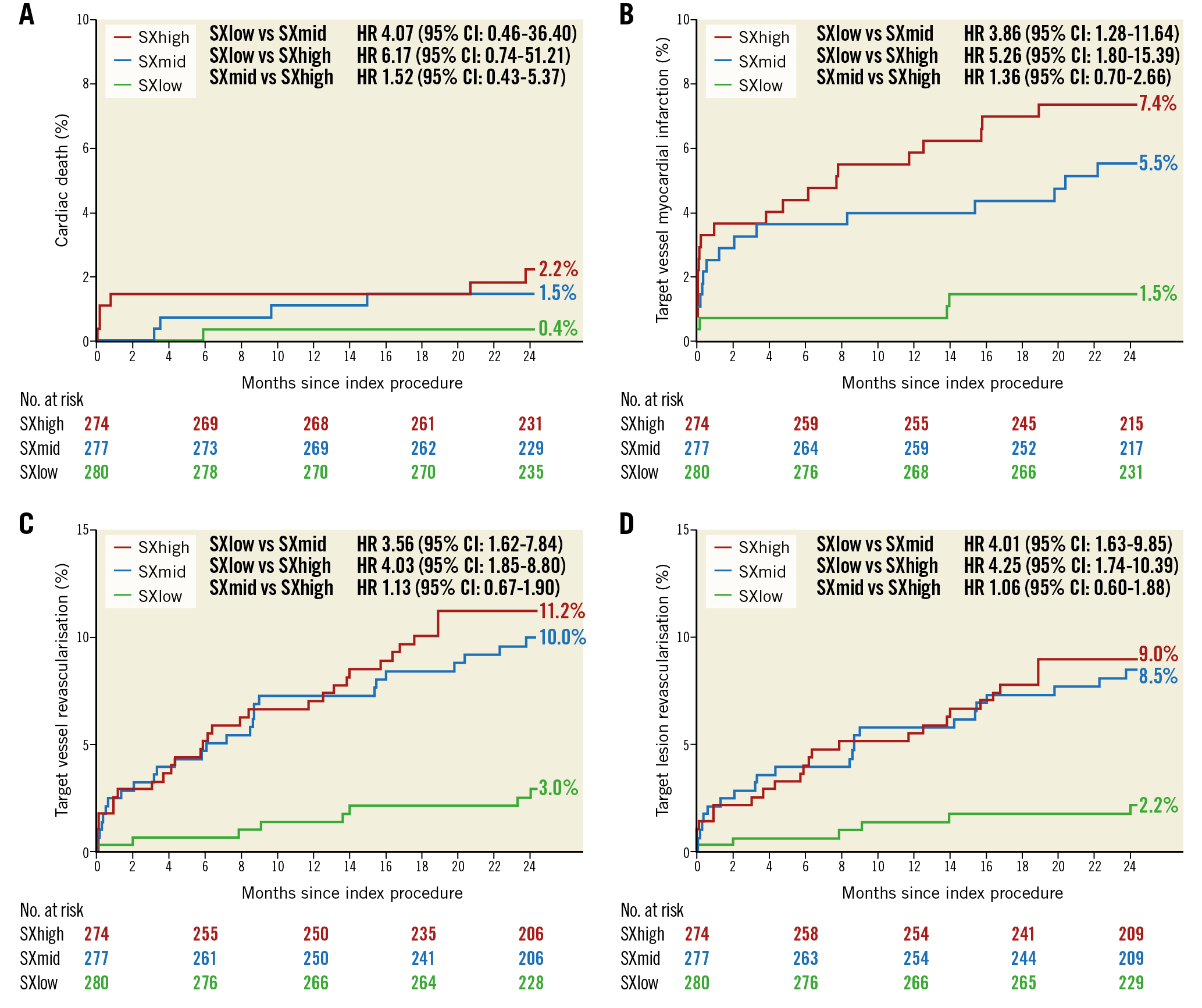
Figure 2. Kaplan-Meier curves for cardiac death (A), target vessel MI (B), TVR (C), and TLR (D) within the Absorb arm.
XIENCE CLINICAL OUTCOMES
Within the XIENCE group, Kaplan-Meier estimates for TVF were 15.6% (SXhigh), 9.3% (SXmid) and 5.6% (SXlow) (p-value for trend p<0.001). Rates were significantly different between the SXhigh and SXlow tertiles (p<0.001) and between the SXhigh and SXmid tertiles (p=0.04), while no statistically significant difference was observed between SXmid and SXlow (p=0.10) (Figure 3A). There were no statistically significant differences for the rates of TV-MI (Figure 3B).
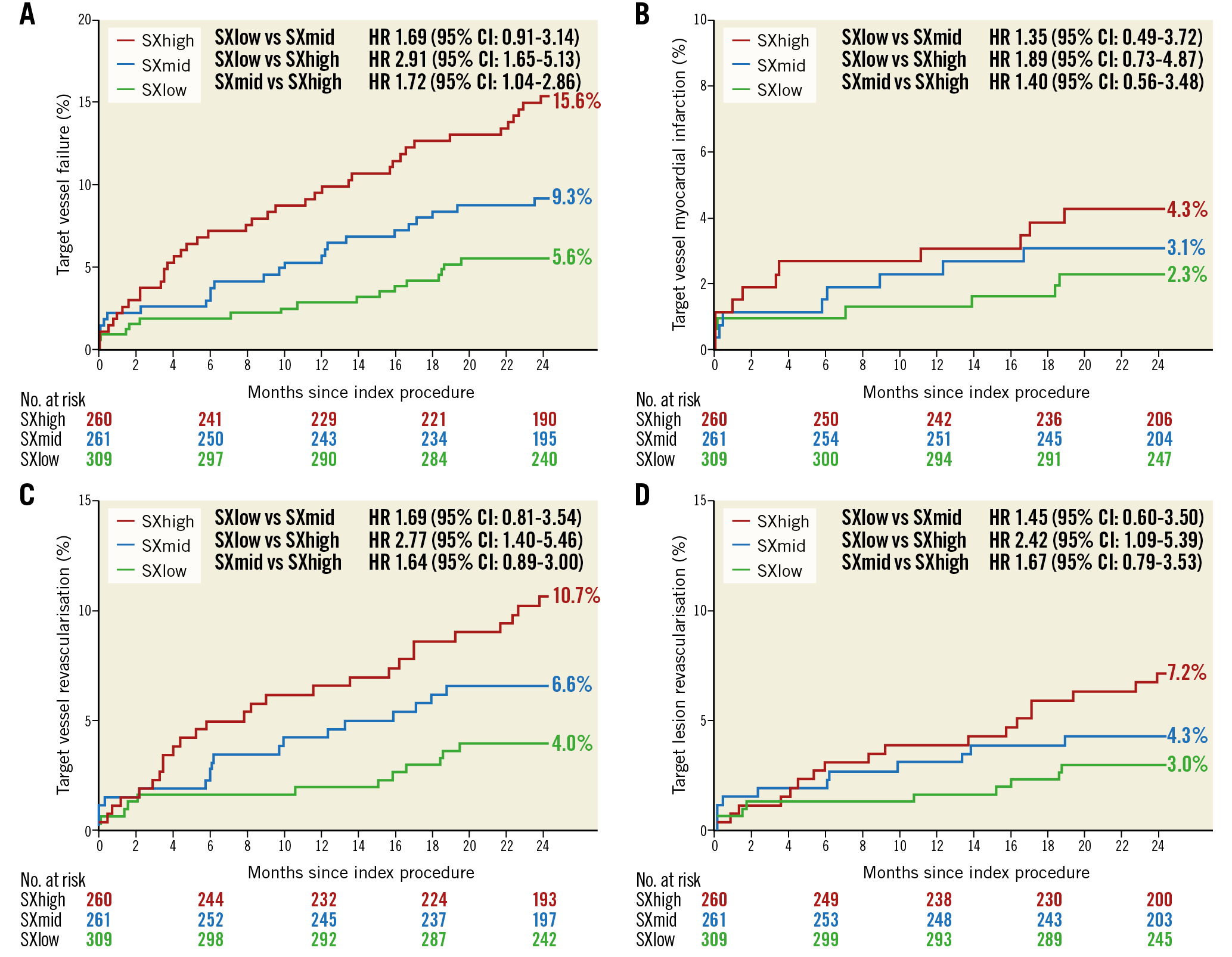
Figure 3. Kaplan-Meier curves for target vessel failure (A), target vessel MI (B), TVR (C), and TLR (D) within the XIENCE arm.
There was a significant difference between the SXlow and SXhigh tertiles for the rates of TVR (p=0.01) and TLR (p=0.07). No statistically significant difference was observed between SXlow and SXmid, or between SXmid and SXhigh tertiles for TLR and TVR (Figure 3C, Figure 3D). The rates of definite ScT were not different across the three SXgroup tertiles (p-value for trend: 0.726). The rates for definite ScT were 0.3% in the SXlow tertile, 0.8% in the SXmid tertile, and 0.8% in the SXhigh tertile.
ABSORB VERSUS XIENCE PER SYNTAX SCORE GROUP
Neither the primary endpoint of TVF nor the other combined endpoint of TLF differed significantly between Absorb and XIENCE in all three SXscore tertiles. Full results of Absorb versus XIENCE per SYNTAX score group are shown in Table 3. The rate of definite device thrombosis differed significantly between Absorb and XIENCE in both the SXmid group (3.3% vs 0.8; p=0.043) and the SXhigh group (3.7% vs 0.8%; p=0.026), while no difference in definite device thrombosis was observed in the SXlow group (1.1% vs 0.3%; p=0.272) (Figure 4). Within the Absorb group, 859 patients were treated with Absorb only, and within the XIENCE group 910 patients were treated with XIENCE only. Analyses of the as-treated population are shown in Supplementary Table 3. Multivariate Cox proportional hazards analyses for the outcomes of TVF and definite device thrombosis are shown in Supplementary Table 4 and Supplementary Table 5, respectively.
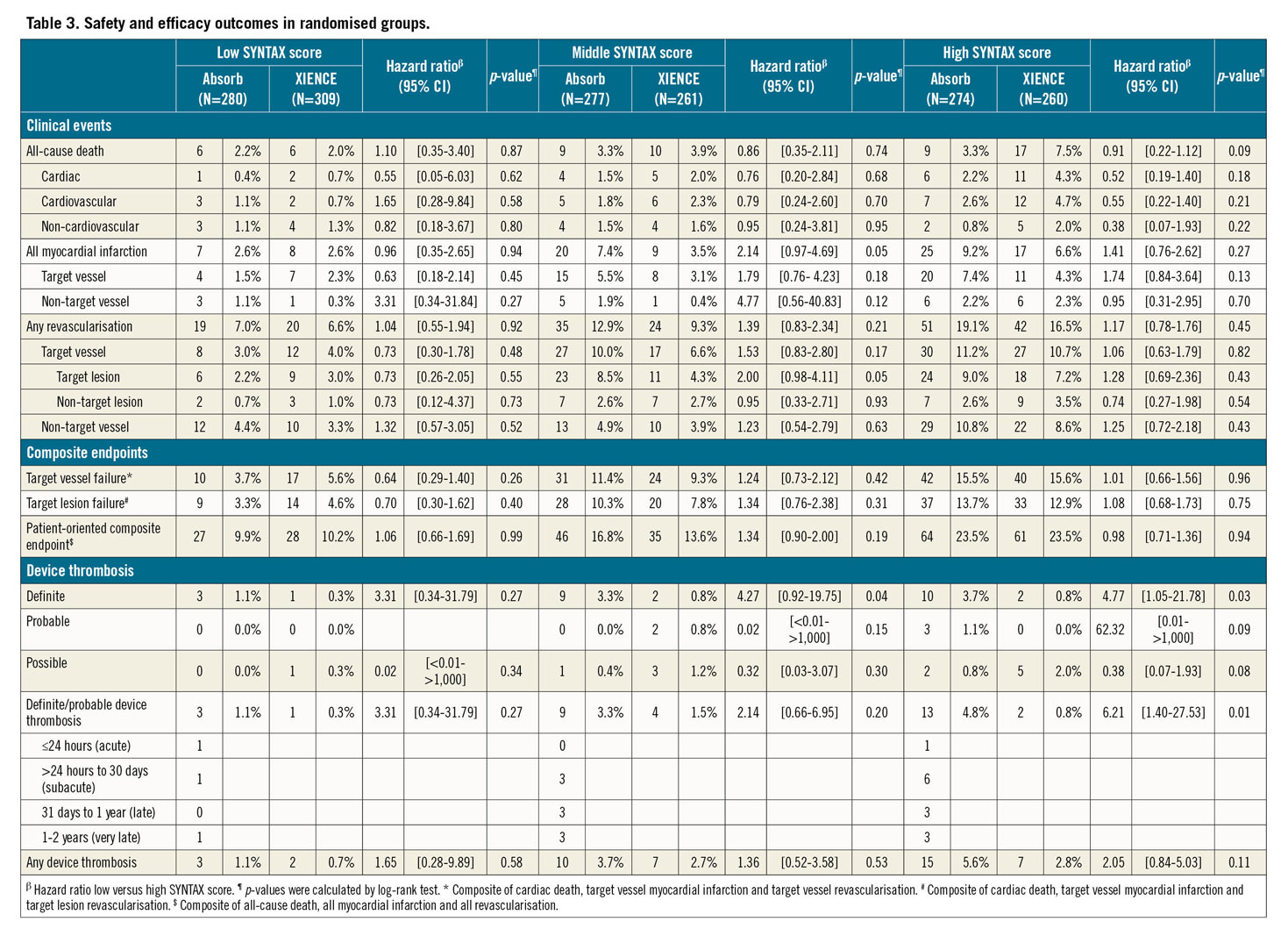
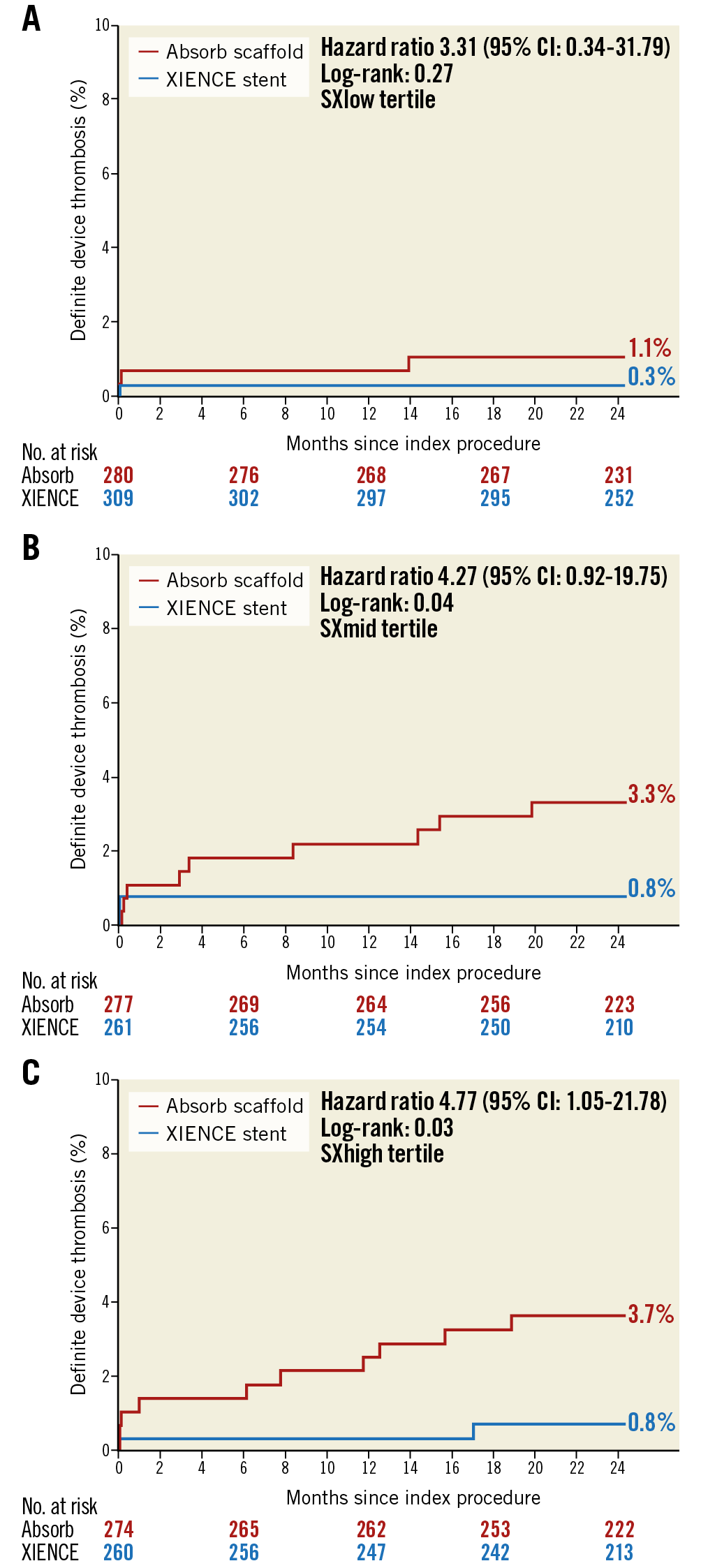
Figure 4. Definite device thrombosis in the three SX tertile groups.
Discussion
The major findings in this AIDA trial substudy are the following.
1) When compared to XIENCE, in the SXlow group, the event rate of TVF was numerically lower within the Absorb group, whereas in the SXmid group the event rate of TVF was numerically higher within the Absorb group. In the SXhigh group, TVF rates were similar between the randomised device modalities.
2) Patients treated with Absorb, and whose SXscore was ≤8 have an acceptable, albeit still threefold higher, thrombotic risk compared with XIENCE at a follow-up of two years.
The current analysis on SXscore identified a patient population (SXscore ≤8) in which Absorb implantation is associated with a lower TLR rate and tolerable ScT rate of 1.1%, which, however, still remains threefold higher compared to patients treated with the XIENCE stent (0.3%). The SXscore, divided into tertiles, proved to be an effective tool to discriminate between groups in routine PCI trials. Most importantly, in patients undergoing PCI with early and newer-generation DES, a higher anatomic angiographic complexity tertile is associated with a gradual increase in rates of clinical events12,13,14,16,17. We have demonstrated that this finding also applies for the overall patient population and the XIENCE group of the AIDA trial. Both analyses demonstrated a significant difference for MI and revascularisations between the SXlow and the SXhigh groups, while no difference was observed between the SXlow and SXmid groups and between the SXmid and SXhigh groups. In the Absorb group, however, no gradual but rather a more abrupt increase in the risk of clinical outcomes was observed. Patients treated with the Absorb in the medium SXscore group (i.e., >8 ≤15) demonstrated a similar rate of clinical events to patients with a high SXscore >15.
The results of this subgroup analysis of the AIDA trial suggest that implantation of the Absorb should only have been considered in patients with relatively simple coronary artery disease, as assessed by an SXscore ≤8. The abrupt increase in events in the Absorb arm observed in this analysis might be due to the insufficient mechanical strength of the device in order to counteract the force of the increased atherosclerotic plaque in patients with an SXscore >811. The lack of mechanical strength could potentially lead to non-embedded, malapposed or even fractured struts, especially in more complex lesions, which can be a potential nidus for neoatherosclerosis, restenosis and/or ScT, MI and revascularisation18. Based on the findings of this subgroup analysis, we recommend not to use a bioresorbable coronary device in routine PCI before short- and long-term safety in low-risk patients and low-complex lesions has been thoroughly evaluated in randomised clinical trials.
Limitations
The present analysis has limitations. First, it is subject to statistical underpowering. Second, as the SXscore was evaluated on the diagnostic angiographic films prior to the procedure, no residual SXscores could be evaluated. Third, acute occlusions in STEMI have been evaluated as total occlusions of unknown duration; this could have led to a possible overestimation of the SXscore. Fourth, due to missing data on ejection fraction or kidney function, no clinical SXscore could be assessed. Fifth, mostly due to logistic reasons, the SXscore was collected in 90% of the patients. However, as the angiograms were collected by research staff blinded to clinical events, bias in collecting the baseline angiographic films is not expected. Sixth, the SYNTAX score showed a core lab reproducibility of <0.6; the reproducibility of this analysis might therefore be limited.
Conclusions
We found no significantly different rates of TVF between Absorb and XIENCE patients. Absorb-treated patients in the SXmid and SXhigh tertiles, however, had an increased risk of device thrombosis when compared to XIENCE-treated patients. The rates of device thrombosis in the SXlow tertile, while still higher for Absorb, are more acceptable than in SXmid and SXhigh score tertiles.
|
Impact on daily practice Implantation of any coronary bioresorbable scaffold in daily clinical care should be reserved for patients with a SYNTAX score ≤8, while implantation in more complex patients, at the moment, should be withheld until future clinical trials, or longer-term follow-up, demonstrate benefits for bioresorbable technology over conventional drug-eluting metallic stents. |
Appendix. Study collaborators
Pier Woudstra, MD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands; Maik J. Grundeken, MD, PhD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands; Karel T. Koch, MD, PhD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands; Jan Baan Jr, MD, PhD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands; M. Marije Vis, MD, PhD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands; Marcel A. Beijk, MD, PhD; Amsterdam UMC and AMC Heart Center, University of Amsterdam, Amsterdam, the Netherlands.
Funding
The AIDA trial is supported by an unrestricted educational grant from Abbott Vascular. The AMC Heart Center has received an educational research grant from Abbott Vascular for AIDA. The Research Departments of the Cardiology Division of the Medical Center Leeuwarden and of the Onze Lieve Vrouwe Gasthuis have received non-study-related unrestricted educational research grants from Abbott Vascular.
Conflict of interest statement
J.P.S. Henriques receives research grants from Abbott Vascular. J.G.P. Tijssen served on the DSMB of the early ABSORB trials, including ABSORB II. The other authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

