Abstract
Atrial septal defects are one of the most common congenital heart diseases in adults that may result in significant left to right shunt. Secundum atrial septal defects can remain unrecognised until adult age and cause haemodynamic changes with or without symptoms. Transcatheter ASD closure is the gold standard; however, in those patients with left ventricular dysfunction there may be increased risk of complications due to acute changes in left-sided pressures. In this review, we discuss the clinical aspects of ASD closure in the setting of LV dysfunction and discuss methods to evaluate risk and strategies to minimise complications.
Introduction
Atrial septal defects (ASD) are one of the most common congenital heart defects with a prevalence estimated at 100 of 100,000 live births1. The current standard of care for secundum ASD in children and adults is transcatheter closure in the setting of appropriate anatomy. Secundum ASD discovered at an adult age may be associated with concomitant left ventricular (LV) dysfunction given the frequent comorbidities in this patient population. In fact, any condition that results in decreased left ventricular compliance, such as hypertension or left ventricular dysfunction, will increase left-to-right shunting and will have an impact on patients during transcatheter closure. The abrupt closure of an ASD in this setting may result in rapid volume and pressure overload of the left heart, resulting in pulmonary oedema and acute left ventricular failure.
In this review, we will discuss transcatheter ASD closure in the setting of left ventricular dysfunction and describe some key strategies in patient evaluation and management.
Pathophysiology of atrial septal defects
ASD are classified according to location, the most frequent being secundum ASD (75-80%), followed by primum ASD (15-20%), sinus venosus (5-10%) and the less common defect of the coronary sinus (<1%). Most primum, sinus venosus and coronary sinus type ASD result in haemodynamically significant shunts and require surgical closure at a young age. Secundum ASD can evolve slowly with a modification of size and shunt flow over time. The direction and magnitude of the flow through the ASD is dependent on the size of the defect and the relative diastolic filling properties or compliance of left and right ventricles.
At birth, pulmonary resistance is high and right ventricle (RV) compliance is low, changing to a low resistance-high compliance circulation with time. When pulmonary to systemic flow ratio exceeds 1.5, the initial volume overload evolves to a pressure overload on the RV which leads to right chamber enlargement with a diastolic septal shift towards the left ventricle (LV) and alters interventricular interaction, resulting in decreasing LV compliance2.
Ventricular compliance can change over time and is affected by coexistent cardiac conditions such as hypertension, ischaemia and congestive heart failure3. Concomitant LV dysfunction causes an increase in LV end-diastolic and left atrial (LA) pressure. In the setting of an ASD, this results in increased left to right shunting and provides a means of “offloading” the less compliant left ventricle. Abrupt closure of this interatrial communication in the setting of transcatheter closure results in an acute increase in left ventricular and left atrial filling pressures as well as myocardial oxygen consumption and may lead to acute decompensation of the left ventricle and pulmonary oedema.
The indications for ASD closure are a ratio of pulmonary over systemic flow >1.5, and enlargement of the right-sided chambers with or without symptoms, in the absence of significant pulmonary hypertension. In the case of elevated pulmonary pressures, closure may be considered if pulmonary vascular resistance is less than 2/3 systemic resistance or pulmonary arterial pressure is less than 2/3 systemic pressure at baseline or responsive to pulmonary vasodilators4,5.
Patients with evidence of left ventricular dysfunction require careful evaluation using echocardiography and invasive haemodynamic assessment prior to transcatheter closure to identify the optimal course of action.
Patient evaluation
ECHOCARDIOGRAPHIC ASSESSMENT
Transthoracic echocardiography (TTE) prior to ASD closure is important to document the size of the defect as well as the presence of right-sided chamber enlargement and right ventricular volume overload in the setting of paradoxical septal motion. Evaluation of pulmonary hypertension and/or right ventricular pressure overload can be assessed by ventricular septal orientation and by using Doppler velocity of tricuspid regurgitation, to estimate pulmonary artery systolic pressure.
In the setting of LV dysfunction, TTE provides an assessment of LV ejection fraction as well as the presence of regional wall motion abnormalities. In addition, evaluation of mitral inflow patterns using peak velocities during diastole (E) and atrial contraction (A), the ratio of E/A and E deceleration time provide an assessment of diastolic function which may be impaired in older patients with an ASD and is frequently associated with significant LV dysfunction. The use of plasma biomarkers such as brain natriuretic peptide may also be helpful to identify those patients with subclinical heart failure symptoms6.
RIGHT HEART CATHETERISATION
In general, right heart catheterisation (RHC) is not necessary prior to ASD closure. However, in LV dysfunction or pulmonary hypertension, RHC is important to evaluate the pulmonary pressures and direction of the shunt. The invasive assessment of LA and left ventricular end-diastolic pressure (LVEDP) prior to ASD closure may predict the occurrence of pulmonary oedema post-closure.
We recommend a full right heart catheterisation including measurement of LA pressure via the ASD, and LVEDP using a pigtail catheter in the left ventricle. For those patients with baseline elevation in LA pressure (>10 mmHg) or LVEDP (>15-20 mmHg), there is likely to be a higher risk of complications if closure is performed without further assessment or intervention. Ideally, such patients should be optimally medically treated with diuretics, antihypertensives, as indicated prior to ASD closure. If the impact of the baseline LA and LVEDP is unclear, a balloon occlusion test with haemodynamic monitoring is recommended7.
BALLOON OCCLUSION TEST
The balloon occlusion test consists of temporarily occluding the ASD with a compliant balloon, such as a sizing balloon used to select device size. This is performed while maintaining a pigtail catheter in the left ventricle to monitor changes in LVEDP as shown in Figure 1. This can also be performed with a catheter in the LA via the ASD or while using a balloon-tipped catheter in the wedge position. The balloon is inflated to “stop-flow”, or no residual shunting by echo colour Doppler evaluation, and maintained in position for 10 to 15 minutes with continuous monitoring of the left-sided pressures. Any significant augmentation (>10 mmHg) of LA or LV end-diastolic pressure or fall of systemic systolic pressure during balloon occlusion is considered a positive balloon occlusion test and mandates further pharmacologic intervention prior to closure or consideration of a fenestrated closure device.
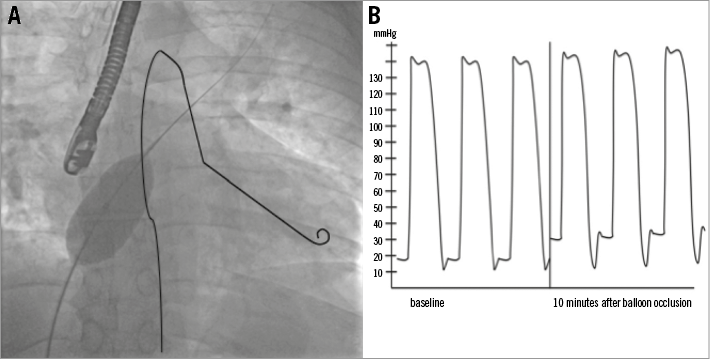
Figure 1. Balloon occlusion test. A) A pigtail catheter has been drawn in the left ventricle to illustrate measurement of left ventricular filling pressures during balloon occlusion test. B) Example of elevation of LV pressures during balloon occlusion.
Management strategies
IDENTIFY AND TREAT REVERSIBLE CAUSES OF LEFT VENTRICULAR DYSFUNCTION
Once LV dysfunction has been detected in a patient with an ASD, efforts should be made to identify an underlying cause. If a reversible cause is found, such as myocardial ischaemia or uncontrolled hypertension, this should be treated first and closure postponed to ensure optimal patient outcomes7. A decision-making management strategy for the treatment of ASD in the setting of LV dysfunction is outlined in Figure 2.
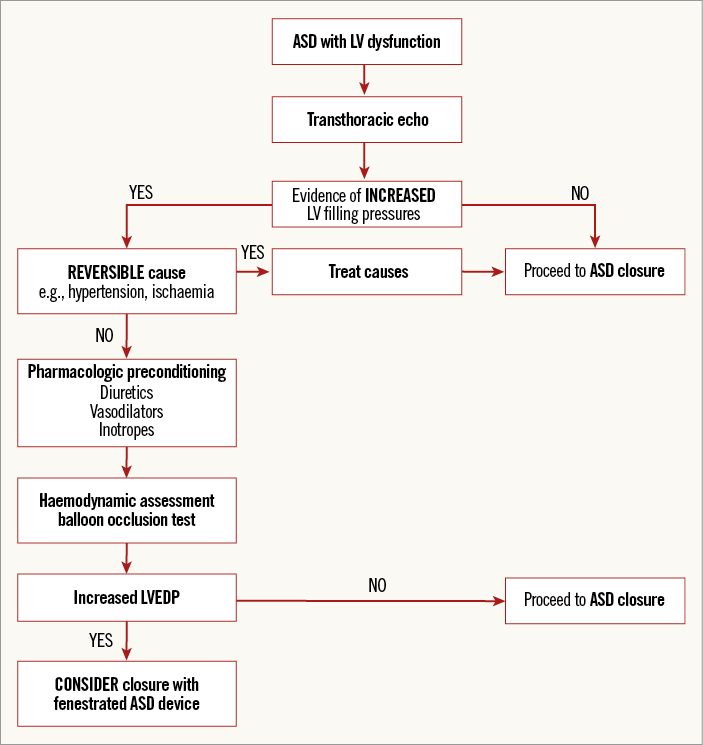
Figure 2. Decision-making process for ASD closure with left ventricular dysfunction. ASD: atrial septal defect; LV: left ventricle; LVEDP: left ventricular end-diastolic pressure
PHARMACOLOGIC PREPARATION
In the absence of a reversible cause, patients with LV dysfunction should be prepared to face the rapid increase in LV filling pressure which will occur during ASD closure. There is a suggestion from a few studies that 48 hours to four weeks of conditioning with diuretics, vasodilators and even inotropes have helped to reduce heart failure and pulmonary oedema. S. Schubert et al presented a cohort of 59 patients considered to be at high risk of pulmonary oedema after closure because of an age older than 60 years: 25% of the patients had increased mean LA pressure during a balloon occlusion test (>10 mmHg). After 48 to 72 hours of intravenous administration of furosemide, milrinone and dopamine, 86% of these patients had a significant decrease of the LA pressure during a follow-up occlusion test, allowing the ASD closure to be performed without complication8. Similar results were shown by C. Gruner et al: 52 patients who were at high risk for acute pulmonary oedema because of age >60 years or arterial hypertension were followed during ASD closure. In this cohort, 27% had a significant increase of left-sided filling pressure during the occlusion test, and subsequently underwent pharmacologic preconditioning with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and diuretics for four weeks. None of the patients developed periprocedural pulmonary oedema during ASD closure9.
FENESTRATED OCCLUDERS
For those patients with large ASD and increased left ventricular filling pressures, ASD closure can be safely performed using a fenestrated closure device. Self-fabricated fenestrated AMPLATZER™ ASD occluders (St. Jude Medical, St Paul, MN, USA) have been described as reducing the risk of pulmonary oedema and heart failure after ASD closure8,10. For such patients, a fenestration of 4 to 6 mm can be made in the occluder to permit some residual shunting following device closure. This fenestration can be closed at a later time, if necessary with a vascular plug if residual shunting is haemodynamically significant and balloon occlusion does not show any significant increase in LV filling pressure11. If a fenestrated device is not appropriate (small ASD, embolic indication), closure should be deferred.
Conclusion
LV dysfunction in the adult patient with ASD should be carefully evaluated to avoid important complications after device closure due to an immediate volume overload on a restrictive LV. Non-invasive assessment of LV systolic and diastolic function and invasive balloon test occlusion with real-time assessment of LV filling pressure is paramount for these patients in order to predict their risk of pulmonary oedema and heart failure. Any reversible cause of LV restriction should be treated first. Pharmacologic preconditioning and fenestrated occlusion devices are therapeutic tools that may help to minimise the risk of heart failure and pulmonary oedema following successful device closure.
Funding
V.X. Tadros was supported by a grant from the Montreal Heart Institute.
Conflict of interest statement
A. Asgar is a consultant for Gore Medical. V.X. Tadros has no conflicts of interest to declare.

