Abstract
Transradial (TR) cardiac catheterisation is thought to be associated with an increased exposure to radiation compared with the traditional transfemoral (TF) access. This paper provides a review of current literature describing these reported associations. Although several studies have reported an increase in radiation exposure to both operator and patient with TR compared with TF access, others have reported findings suggesting no significant difference, even reporting decreased exposure with TR access. Ultimately, increased radiation exposure appears likely with TR access; however, in consideration of the many benefits associated with TR access, radiation exposure remains only one of many considerations when deciding between routes of access.
Introduction
Diagnostic and interventional cardiac catheterisation represents an important source of radiation1. While transfemoral (TF) access has been the mainstay of catheterisation procedures, the transradial (TR) route has become increasingly popular due to benefits in cost, patient outcomes, and patient comfort2-5. Numerous studies have demonstrated a reduction in vascular complications with TR access, improvements in ambulation time, length of post-procedure hospital stay, and simplified same-day discharge6. Despite these advantages, TR is estimated to be used in only 6-12% of diagnostic coronary procedures and percutaneous coronary interventions (PCI) worldwide7-9.
One explanation for the slow adoption rate of TR is the perceived concern about an association between TR catheterisation and increased radiation exposure to both operator and patient10-13.This paper will review the most pertinent contemporary literature and describe the risk of radiation exposure as well as protective strategies for both operator and patient with TR access.
Radiation dose basic principles
Protection from radiation exposure is driven by what is known as the ALARA principle, the goal of maintaining exposure at a level that is “as low as reasonably achievable”14 by increasing the distance from the radiation source, by decreasing the duration of exposure, and by the implementation of shielding equipment.
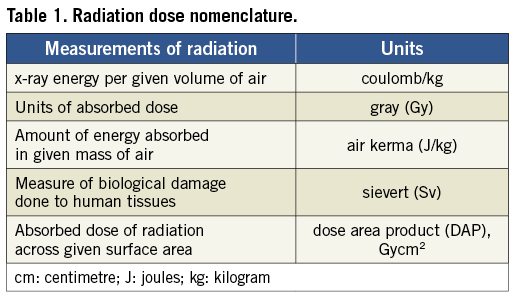
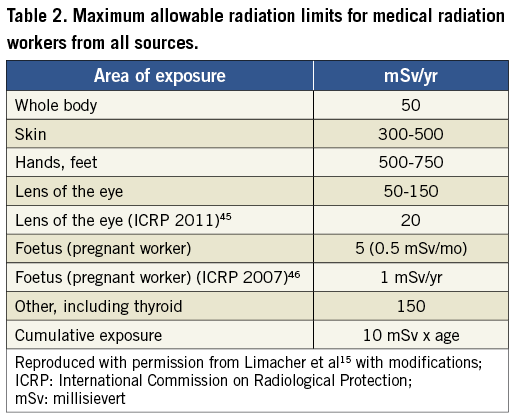
Radiation can be expressed in a variety of ways. Concentration of x-ray or gamma-ray ionising energy in a given volume of air is expressed as coulombs/kg15. Units of absorbed dose, expressed as gray or Gy, take into account the amount of energy imparted to a specific point by mass. Air kerma is the amount of energy absorbed in a given mass of air, expressed as joules/kg. Finally, the dose equivalent, or a measure of the biological damage done by radiation to human tissues, is expressed as sievert (Sv)15. Absorbed dose is often expressed as a dose area product (DAP), which is a measure of the absorbed dose of radiation spread over a given surface area of exposure16. DAP allows for the estimation of dose to the irradiated tissue and may therefore be the best indicator for cancer risk. Fluoroscopy time (FT) is the most readily assessed and reported measurement of radiation exposure; however, it is an indirect measure of radiation (Table 1 and Table 2).
Patient exposure
Several observational and randomised studies suggest differences in radiation exposure to patients between TR and TF catheterisation. The majority of this data infers increased radiation exposure to both patient and operator through increased fluoroscopy time without data to support direct increased exposure (Table 3). The paucity of quality, randomised data in the literature has confounded efforts to identify trends in exposure associated with the TR access definitively.

Despite these considerations, the majority of studies reviewed concluded that TR access was associated with higher patient exposure rates (Table 4, Figure 1). However, the radiation risks of the TR access to the patient must be carefully weighed against its benefits, namely its reduced rates of complication.
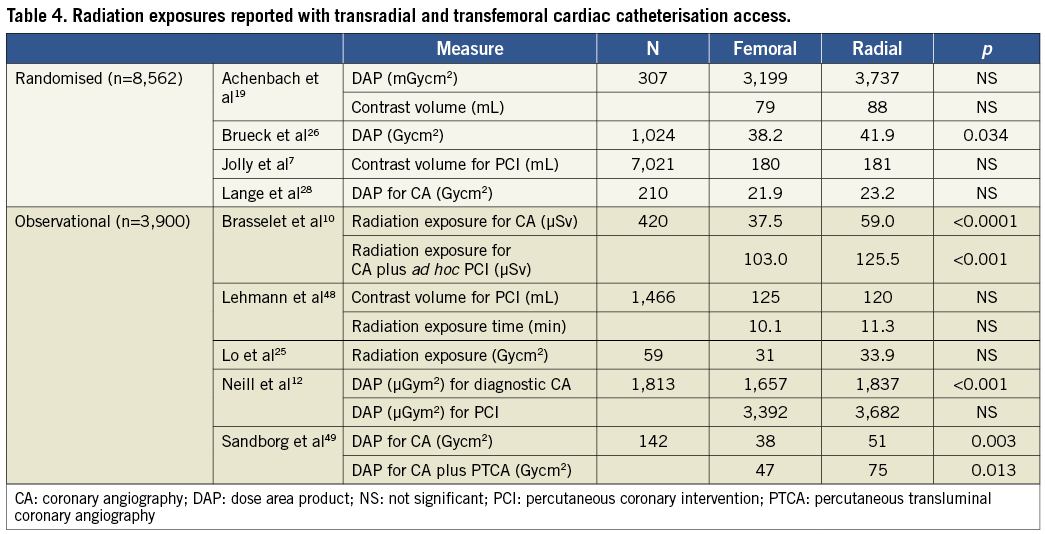
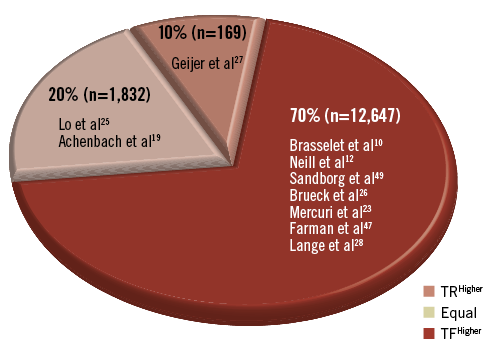
Figure 1. Percentage of studies explored that reported patient radiation exposures. TF: transfemoral; TR: transradial
Moreover, the one-time increase in radiation exposure to the patient remains far below the threshold for deterministic effects to be seen12,17,18. Placed into perspective, Neill et al reported a difference in diagnostic studies between TR and TF access of just 0.4 mSv, the equivalent of 20 chest x-rays. Additionally, the increase in lifetime risk for induction of cancer with the TR approach amounted to just 0.002%12.
Most studies reported significant differences in FT between TR and TF access, highlighting the increased time that was necessary to navigate the catheter into the ascending aorta to intubate the coronary artery ostia; cineangiography, which delivers a higher dose of radiation, is not seen to differ10,19. Importantly, many studies reported FTs as alternative measures of radiation exposure with the assumption that increasing FT correlates with increased exposures. However, FT does not include aspects of the procedure such as cine acquisition and is not a reliable predictor of radiation dose20,21. These findings suggest that operators should be cognisant of FT and the need for cineangiographic imaging capture during TR procedures.
Several studies noted a significantly higher body mass index (BMI) of patients in the TR comparison group10,12,22,23, presumably due to greater risk or difficulties encountered in accessing the femoral artery in these individuals. One study reported a positive correlation between BMI and DAP (correlation coefficient 0.42, p<0.001)12. Secondly, another reported the thickness of the patient to be the most dominant factor in determining radiation scatter dose values24. These findings suggest that the predilection for TR catheterisation in heavier individuals may further skew results towards greater exposure associated with this route. Despite these concerns, several studies2,19,25,26 reported no significant differences in BMI between TR and TF groups and the extent to which such factors influence exposure trends remains unclear.
Operator exposure
Compared with literature on patient radiation exposures, little data exist on operator radiation exposures with TR catheterisation. Nonetheless, published studies have reported strong correlations between patient and operator exposure10,27, with one study reporting correlation factors of r=0.68 (p<0.0001) and r=0.61 (p<0.0001) for CA and CA followed by ad hoc PCI, respectively10. Therefore, it is reasonable to assume that an increase in patient exposure will result in an increase in operator exposure.
Brasselet et al reported an increase in radiation exposure from 13.0 to 29.0 μSv with TR access when compared with TF access in coronary angiography (CA) procedures (p<0.0001), and a similar increase in CA followed by ad hoc PCIs: 41.0 vs. 69.5 μSV (p=0.018)10. Overall, there was an 82.7% increase in operator radiation exposure for radial CA, and a 38.1% increase for radial CA followed by ad hoc PCI.
Lange et al reported an increase in operator radiation exposure of 100% (p<0.001) for TR diagnostic procedures, and an increase of 51% for TR PCI (p<0.05)11. When comparing TF to TR approaches to diagnostic CA, FT increased from 1.7 to 2.8 minutes (p<0.001) and DAP increased from 13.1 to 15.1 Gycm2 (p<0.05) for coronary interventions; however, there was no significant difference in either. Total radiation exposure for CA followed by PCI increased from 110 to 166 μSv (p<0.05)11. Of note, the authors of this study utilised a 7” shield flap attached to a side shield for femoral cases, a shield flap which was not utilised for radial cases due to the potential hindrance in TR access. The upper shield flap, normally an extension of a 0.5 mm lead pivotal side shield utilised during TF shielding, was flipped downward to provide maximal access to the TR access site. The extent of the influence of this additional protection on the exposures reported is unknown.
However, in a more recent study by Lange et al28, the shield flap was uniformly folded down for all cases. They again reported increased operator radiation exposures associated with TR access (20.9 vs. 15.3 µSv, p<0.001). The difference was largely attributed by the authors to closer operator proximity to the radiation source required for TR access.
Mann et al reported increased external whole body dose to the operator associated with TR over TF access (13.5 vs. 8.8 mrem/case, p<0.01). However, with the addition of a movable floor shield to standard safety equipment, TR radiation decreased to levels below those of TF access (3.3 vs. 8.8 mrem/case, p<0.01)29.
In the largest study to date, reviewing over 5,954 diagnostic catheterisations, Mercuri et al23 studied 16 high-volume operators with extensive experience in both TR and TF approaches. They employed a multivariate regression model to account for a variety of factors including BMI, sex, age, the presence of a fellow, and previous CABG. The study concluded that radiation exposure varied significantly from one operator to the next, but across the board exposure was greater with TR access (FT 3.82 vs. 5.46 min, p<0.001; log air kerma 6.28 vs. 6.49 mGy, p<0.001). Interestingly, they found that for each operator the difference in exposure between TR and the TF access was about equal, downplaying the impact of varying operator experience on the perceived trend towards increased exposure with TR access. Rather, increased exposures appear inherent to TR access. Furthermore, the variability in radiation exposure between operators was seen to be greater than that between access sites for a given operator. In the light of these findings, concern over the apparent increase in radiation risk inherent to TR access may be supplanted by an emphasis on adequate training of highly skilled operators, regardless of the access site chosen.
Mercuri et al concluded that, while exposures are higher with TR access, the increase remained below the level at which deterministic effects (2-Gy) may be seen. The cumulative effects to the operator, however, are less certain. Assuming a 20-year career, the authors found the increased exposure would amount to four additional years of exposure were the operator to perform TR procedures exclusively. Importantly, the authors conceded that their study was not designed to investigate operator dose, and the full cumulative effects on operators are not well known23.
Right vs. left radial approach
Several studies have illuminated differences in radiation exposure and FT between the left and right radial access (Table 5). Findings of prolonged FT with the right vs. left approach may be secondary to greater subclavian artery tortuosity and the associated imaging needed to navigate this anatomy with right radial access. However, trends towards increased exposure with right access may be offset by the fact that right-sided access is more easily accessed by traditional cardiac catheterisation laboratory set-ups that situate the operator on the right side of the patient.
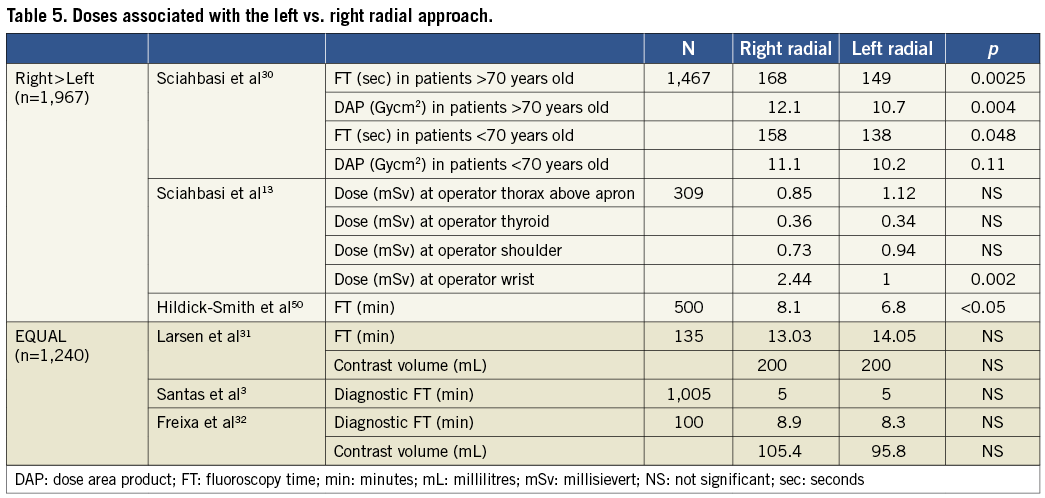
In the TALENT study, Sciahbasi et al compared left versus right TR approaches for coronary angiography. They found that for patients ≥70 years old, the left TR approach was associated with a shorter FT (149 vs. 168 seconds, p=0.0025) and less DAP (10.7 vs. 12.1 Gycm2, p=0.004) compared with the right. However, in patients <70 years old, there was only a trend towards such results30. The average patient weight in this study was 78 kg. Since large body habitus significantly impacts on performance of the left radial approach the relationship between body weight and exposure from left vs. right radial catheterisation is unknown at the present time.
Sciahbasi et al additionally explored operator radiation exposure associated with left vs. right TR approaches and found no significant differences at any of the following dosimeters: respectively, thorax above the lead apron (1.12 vs. 0.85 mSv, p=0.33); thyroid (0.34 vs. 0.36 mSv, p=0.87); shoulder (0.94 vs. 0.73 mSv, p=0.27). However, they reported significantly higher exposures at the level of the wrist associated with right vs. left TR access (2.44 vs. 1 mSv, respectively, p=0.002). Radiation exposures were undetectable for the thorax under the lead apron for both approaches13.
In contrast, a study by Larsen et al found no significant difference between FT (14.5 vs. 13.03 min, p=0.8162) or contrast volume (200 vs. 200 mL, p=0.87) between left and right TR access, respectively31. Similarly, Santas et al reported no significant differences in diagnostic FT between left and right radial access (5 vs. 5 min, p=0.2)3. Lastly, a recent study of octogenarians by Freixa et al reported more frequent subclavian tortuosity with right vs. left access, but no difference in fluoroscopy time (8.9 vs. 8.1 min, p=0.704) or contrast volume (105.4 vs. 95.8 mL, p=0.217)32.
Radiation protection devices
Traditionally, standard protective garments include a 0.5 mm lead apron, effective at blocking up to 95% of radiation33, a lead thyroid collar, eye-protection glasses, leaded glass shields projecting from the ceiling, and lead glass or fabric drapes under the bed to the floor to prevent scatter from reaching the physician. Several groups have devised equipment in addition to standard protection in the hope of further minimising operator radiation exposure during TR procedures (Figure 2).
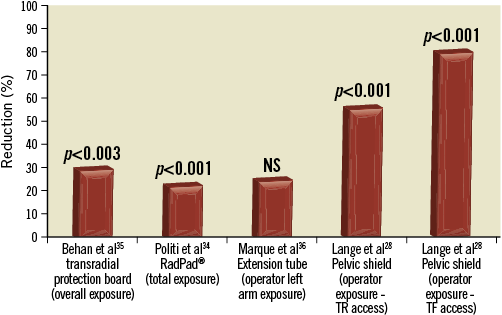
Figure 2. Percentage reduction with various shielding. NS: not significant; TF: transfemoral; TR: transradial
RADIATION PROTECTION DRAPES
The RadPad® (Worldwide Innovations & Technologies, Overland Park, KS, USA) is a flexible, lead-free disposable radiation drape designed to produce significant reduction in scatter radiation during fluoroscopy procedures. In a randomised trial exploring the efficacies of the RadPad® during TR cases, Politi et al reported a significantly decreased total radiation exposure to the operator (282.8 vs. 367.8 μSv, p<0.0001). Overall, they reported a 13-34% absolute reduction in mean radiation exposure at all locations of the body - wrist, chest, thyroid, and eye level. The RadPad® was used in addition to conventional shielding tools and was shown to add additional radiation protection to the operator without increasing FT (Figure 3)34.
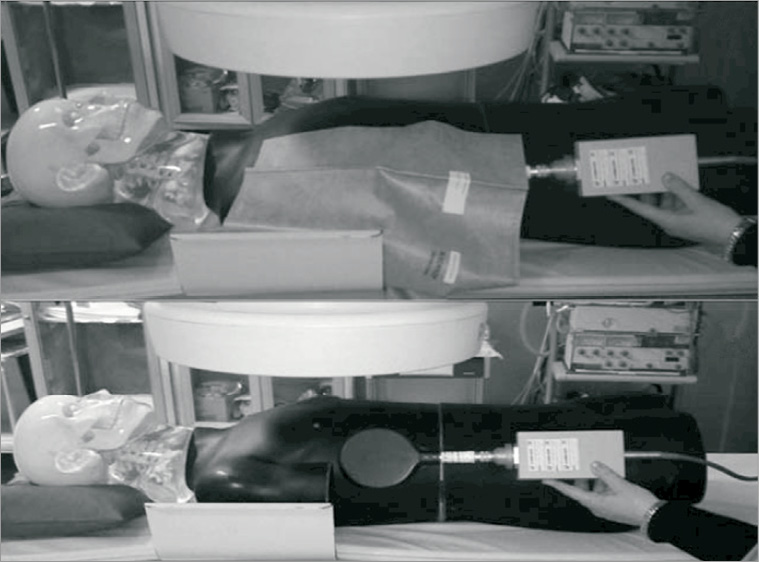
Figure 3. Simulation of radiation exposure with (A) and without (B) the RadPad®. Image reproduced with permission from Politi et al34.
In an effort to elucidate further the role of lead shields in TR catheterisation, Lange et al28 conducted a study utilising a pelvic lead shield. They found the shield lowered operator exposure for TR access from 20.9 to 9.0 µSv (p<0.0001), and for TF access from 15.3 to 2.9 µSv (p<0.0001). The authors concluded that, with the implementation of their pelvic lead shield, an operator would be able to perform four times the number of femoral cases and twice the number of radial cases for the same amount of radiation exposure. With the use of the pelvic lead shield, this study has shown very low radiation exposures can be achieved, especially with transfemoral catheterisation.
PROTECTION BOARDS AND EXTENSION DEVICES
Behan et al developed a transradial radiation protection board (TRPB) to be used in addition to conventional protection equipment. They found the TRPB allowed for a significant reduction in operator radiation exposure (28 vs. 19.5 μSv, p=0.003) for control vs. TRPB, respectively. For both diagnostic CA and ad hoc PCIs, there were no significant changes in total FT, procedure duration, or contrast load (Figure 4)35.

Figure 4. Transradial radiation protection board. Image reproduced with permission from Behan et al35.
Based on the principle that operator exposure maintains an inverse-square relationship with the distance from the source of radiation, Marque et al hypothesised that the implementation of a 30 cm extension tube would further decrease operator exposure during TR angiography, similar to the distance a femoral approach would allow. However, they found only a trend towards lower operator exposure at the level of the lower left arm (28.7 vs. 38.4 μSv, p=0.0739). No significant difference was perceived at the level of the thorax36.
Learning curve
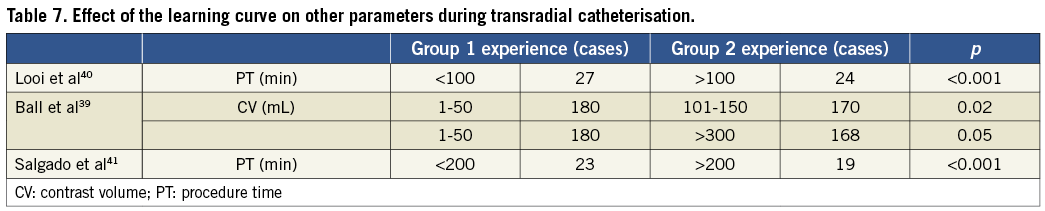
Several studies have suggested the minimal number of cases to achieve modest proficiency in the TR technique, ranging from 50 to 20037,38. Although the present data on the impact of the learning curve with TR CA on radiation exposures are scarce, the findings strongly suggest a significant influence indeed12,39-41.
In a study of 1,001 patients, Looi et al compared radial experts, defined as interventionalists with experience of over 100 TR procedures with non-experts. For diagnostic CA with or without ad hoc PCIs, the non-expert group had significantly higher procedure times (27 vs. 24 min, p<0.001) and longer fluoroscopic times (6.2 vs. 5.3 min, p<0.004) than the radial expert group. However, after three months, these differences were no longer apparent. At six months, there were significant reductions in both FT (7.3 vs. 6 min, p=0.04) and procedure time (30 vs. 26 min, p=0.04) within the non-expert group.40
Ball et al conducted a study including 1,672 patients and 28 operators, the latter being categorised by their TR experience into groups of 1-50, 51-100, 101-150, 151-300. The group with >300 cases was designated the control group. Significantly longer FTs were associated with the 1-50 group compared with the 101-150 group (15 vs. 13 min, p=0.04) and compared with control (15 vs. 12 min, p=0.02). In addition, greater contrast volumes were associated with the 1-50 group compared with the 101-150 group (180 vs. 170 mL, p=0.02) and compared with control (180 vs. 168 mL, p=0.05). Their findings suggested decreases in contrast volumes and FTs with greater experience, with a substantial absolute change observed after 50 cases39.
In a study encompassing over 1,800 cases, Neill et al imposed a transition phase to account for the influence of a learning curve on operator exposure. They found that for diagnostic procedures FT decreased from the transition phase to the default radial phase, 5.12 vs. 4.21 min (p=0.03), but there was no significant change in DAP. For interventional procedures, FT increased from 10.51 to 12.14 min (p=0.02) with no significant change found in DAP12.
Most studies exploring the role of the learning curve did not report actual exposure, but instead procedure duration and FTs, parameters which can both only be assumed to imply increased exposure (Table 6 and Table 7).
Areas of uncertainty and future challenges
The impact of the TR approach and radiation exposure on catheterisation staff has not been explored. While assumptions may be made regarding increased exposures and prolonged FT and cine-acquisitions, for obvious reasons, it is important to characterise doses using dosimeters on staff members apart from the operator. Additionally, the impact of the type of source equipment utilised in TR catheterisation is as yet unknown. Some data have suggested significantly higher dose rates associated with image-intensifier units compared with flat-panel detectors42, while others have reported no differences between them43. Perhaps modern equipment, with its high image quality, may allow inexperienced and improperly trained operators a greater margin within which to practise poor technique. Lastly, medico-legal implications of performing TR procedures which afford reduced complications and access-site bleeding while delivering increased radiation dose to the patient are uncertain.
The reporting of radiation exposures during interventional cardiology procedures remains a relatively scarce practice. One estimate places only about 4% of studies between 1996 and 2010 as having reportable radiation exposures44. Until a greater emphasis is placed on exploring maximal radiation safety with the radial access, this approach may not gain widespread acceptance within the United States.
Summary
The majority of studies suggest that TR coronary angiography is associated with a small but increased radiation dose to the patient and the operator compared to the TF approach. However, much of the data is observational, with the majority of studies presenting findings of fluoroscopy time, a poor indicator of radiation exposure; there remains a need for definitive randomised studies. While the one-time increase in exposures to patients with TR access appears minimal, various methods may be employed to reduce cumulative exposures to operators. In addition to general radiation “best practices”, the left radial approach, increased operator experience and additional shielding will significantly reduce radiation doses. Further research is needed to determine the impact of new imaging equipment, dedicated radial catheters and techniques for radiation dose reduction.
Conflict of interest statement
L. Van-Thomas Crisco is founder of and owns interest in Radial Assist, LLC. The other authors have no conflicts of interest to declare.

