
Abstract
Aims: We aimed to examine the relative performance of the new COMET wire from Boston Scientific (BS), and the established technology from St. Jude/Abbott Vascular (SJ).
Methods and results: We compared simultaneous readings from pairs of wires. Patients were randomised to one of three groups: BS/BS, SJ/SJ, or SJ/BS. The last group was sub-randomised to specify the type of wire that would be passed first. After pressure equalisation at the guide catheter, we recorded paired observations in sequence: (a) distal to proximal pressure ratio at baseline, (b) FFR at maximum hyperaemia, and (c) pressure on withdrawal into the guide catheter to quantify “drift”. We randomised 106 patients, yielding 288 sets of paired recordings (BS/BS=90; SJ/SJ=90; SJ/BS=108). Drift was recorded from 208 vessels (BS=105; SJ=103). All wires were successfully advanced to their desired positions in the coronary vasculature. The mean (±SD) differences for the randomised pairs were similar: BS/BS=0.0016 (0.023); SJ/SJ=0.002 (0.03); SJ/BS=0.0013 (0.028). The primary outcome tested the hypothesis that the absolute magnitude of the difference (irrespective of sign) observed in the SJ/BS pairing would be similar to that in the SJ/SJ group. The median (IQR) values were SJ/BS=0.015 (0.01-0.03); SJ/SJ=0.01 (0.00-0.03); p=0.61. The drift, expressed as the median (IQR) difference in Pd/Pa from 1.0 (irrespective of sign), was similar: BS=0.02 (0.01-0.05); SJ=0.02 (0.01-0.04); p=0.14.
Conclusions: We found no significant difference between these wires in terms of safety and performance. Clinical Trials Registration: NCT02578381. https://clinicaltrials.gov/ct2/show/NCT02578381
Abbreviations
±: plus or minus
BS: Boston Scientific
CI: confidence interval
CRF: case record form
FFR: fractional flow reserve
GC: guiding catheter
IQR: interquartile range
IRAS: UK Integrated Research Application System
Pa: aortic pressure - measured with a guide catheter
PCI: percutaneous coronary intervention
Pd: distal pressure - measured in a coronary vessel
PW: pressure wire
SD: standard deviation
SJ: St. Jude
UK: United Kingdom
Introduction
Coronary atheroma has the potential to impact on blood flow and result in myocardial ischaemia, particularly at times of increased demand. The measurement of fractional flow reserve (FFR) is established as the reference standard for the assessment of the functional significance of coronary artery disease1,2. The FFR is defined as the ratio of the pressure distal to a stenosis (Pd) relative to the pressure proximal to the stenosis (Pa) during hyperaemia induced by a vasodilating agent. There is increasing recognition that the clinical target of coronary revascularisation should be the resolution of ischaemia3. The clinical value of FFR-based decision making in patients already committed to PCI on the basis of a diagnostic angiogram has been established in a number of randomised controlled trials and is supported by international guidelines4-6. Furthermore, a growing number of observational studies have demonstrated a consistent effect upon patient management (with change in 22-48% of cases) when FFR is used at the time of the baseline diagnostic angiogram7,8.
The invasive measurement of FFR is achieved by measuring the pressure in a coronary vessel, distal to the diseased area, usually with a special pressure wire (PW) that can subsequently (if required) be used for the delivery of percutaneous intervention devices. There is a paucity of data comparing the relative performance of different PW systems, though the development of an alternative approach with pressure measurement through a microcatheter (advanced over a traditional angioplasty guidewire) has prompted some comparative studies9,10.
Boston Scientific has recently developed the COMET™ PW system (Boston Scientific, Marlborough, MA, USA) which is now available for routine clinical use. This wire is being used in the multicentre randomised controlled trial RIPCORD 2 which compares a strategy of systematic FFR assessment of all coronary vessels (of sufficient calibre to be a revascularisation target) with a traditional approach – using angiographic assessment alone11.
The COMET wire uses a fibre-optic pressure sensor system, in contrast to the St. Jude/Abbott pressure wire (St. Jude Medical, St. Paul, MN, USA) which uses a piezo-electric pressure sensor. The aim of this study was to assess, in a novel and randomised manner, the clinical performance and consistency of pressure measurements of the COMET wire (BS) compared to the established technology of St. Jude (SJ) (now Abbott Vascular).
Methods
GENERAL
The conventional approach to this type of investigation is to test only the experimental and reference product, ideally in a series of paired simultaneous measurements. The limitation of this approach is that it ignores the reality that some variation would be present even with PW technology of the same type. We designed a randomised study to test the primary hypothesis, “Is the magnitude of the difference observed in paired simultaneous recordings of coronary pressure any different with the use of BS and SJ PW when compared to two SJ wires?” This design also provides useful information about the measurement consistency and performance of the individual wire types.
The design of the study was approved by the UK National Research Ethics Committee (Integrated Research Application System [IRAS] reference 188995). All patients were provided with an approved patient information sheet and were allowed time for reflection and questions before providing written informed consent to participate in the study.
CONDUCT
Patients were recruited at two UK centres (Southampton and Liverpool). We approached adult patients who were scheduled for coronary PW examination as part of their routine care. We excluded patients with: presentation in the context of an ST-elevation myocardial infarction, previous coronary artery bypass grafting, significant valvular heart disease, atrial fibrillation, pregnancy, renal dysfunction with a serum creatinine of greater than 180 µmol/l (2.04 mg/dl), or known intolerance to adenosine. Eligible and consenting patients were randomised in the cardiac catheterisation laboratory after angiography had confirmed the clinical requirement for and anticipated safety of a PW procedure with haemodynamic stability, a stable guide catheter (GC) position and no aorto-ostial disease.
RANDOMISATION
Patients were randomised into one of three groups: BS/BS, SJ/SJ, or SJ/BS. The last group was sub-randomised to specify the type of wire that would be passed first to control for the possibility that the presence of an existing wire might affect either the ability to pass a second, or the measurements recorded. If more than one vessel was to be examined, the same randomised allocation was used for all PW activity in that patient. Randomisation was performed using a secure website that required registration of the patient (using a unique trial number) before release of the group allocation. Randomisation was stratified by centre and based on tables prepared by an independent statistician. Randomisation was block stratified in blocks of 2, 4 and 6 with random variation of the block size.
PROCEDURE AND DATA COLLECTION
The use of 6 Fr guiding catheters was mandated to optimise the quality of phasic pressure recording at the tip of the GC. The protocol demanded that pressure recordings were made after the selective administration of intracoronary nitrate in each target vessel system. Simultaneous equalisation of the aortic and PW tracings was performed with both PW sensors at the GC tip before wires were advanced, sequentially, down the coronary vessel. The wires were adjusted such that the sensor positions were at an identical location in the appropriate region in the target vessel. This co-location was confirmed in two orthogonal radiographic projections.
Pressure readings were taken after the immediate effects of intracoronary nitrate and radiographic contrast had subsided. Simultaneous readings were then taken from the wires to record the baseline distal to aortic pressure ratio (Pd/Pa). The FFR was then measured from both wires after maximum hyperaemia had been induced by an intravenous infusion of adenosine to achieve a “steady state”. When the wires were withdrawn from the vessel, the observed “Pd/Pa” was recorded in order to allow quantification of any deviation from the original value of unity (often referred to as “drift”).
Data were collected in a bespoke case record form and transcribed to a secure electronic database. All patients were tracked from randomisation for 24 hours or to hospital discharge (whichever occurred sooner) for the occurrence of adverse events. Adverse events were reviewed by the principal investigators at the two sites who reached a consensus decision about seriousness and any potential causal relationship to the pressure wire procedure. Because of the small size of the study, no formal interim safety analysis was planned. The protocol afforded the option to suspend recruitment and to invite external review in the event of an adverse event rate above that suggested by historic pressure wire studies.
POWER CALCULATION AND STATISTICAL METHODS
The study planned to randomise about 100 patients. We assumed that, if the average number of epicardial vessels assessed in each patient was 1.5, then this would yield 150 individual sets of paired vessel analysis for both baseline Pd/Pa and FFR – or 300 sets of paired readings, 100 in each group. The power calculations were made on this latter figure.
Using a precision method (most applicable to the Bland and Altman method), for an observed difference in means of 0.01, the 95% confidence interval (CI) for this point estimate would be 0.0041-0.016. For a more substantial point estimate difference of 0.07, the comparable 95% CI would be 0.06-0.08.
For the primary outcome, we assumed that the observed mean absolute difference in the paired readings of the reference wire (SJ) would be 0.01 with a standard deviation of 0.03. Using conventional power calculations (designed for parametric data), a sample size of 96 in each group would afford 90% power to detect a difference of 0.0135 between the means of the two groups. As the primary outcome examined the absolute difference, irrespective of sign, non-parametric tests would be required and, in general, this can increase the required sample size by 15%. With non-parametric methods, for the same effect size, recruitment of 94 patients in each group affords 85% power.
In the calculation of the difference between individual paired observations, the recording from the wire placed second was subtracted from the wire placed first except in the group with discordant wires (SJ/BS) where the calculation was SJ–BS irrespective of placement sequence (which was dictated at randomisation).
All analyses were performed on an intention-to-treat basis using SPSS Statistics, Version 24 (IBM Corp., Armonk, NY, USA). The non-parametric power calculation was performed using the G Power method (http://www.gpower.hhu.de/). Descriptive statistics are presented as means with the standard deviation (SD) or medians and interquartile range (IQR) for non-parametric data. The agreements between individual pairs of wire types are presented using the method of Bland and Altman12. For the primary outcome, we compared the absolute differences in simultaneous paired readings between the groups (irrespective of sign) using a Mann-Whitney test. A p-value of 0.05 was assumed to indicate statistical significance.
The magnitude of drift was compared, for the two wire types, using information derived from readings made on withdrawal from individual coronary vessels, without reference to the randomised allocation (“all BS” versus “all SJ”). Drift was calculated as the difference in the observed “Pd/Pa” from unity, irrespective of sign. The median values for the two wires were compared using a Mann-Whitney test.
Results
A total of 106 patients were randomised between March 2016 and November 2016. There were only two adverse events. In one case, introduction of the coronary GC caused a dissection that was identified before passage of a pressure wire. This was treated with stent implantation without sequelae. In the second case, the administration of adenosine provoked bronchospasm that required treatment with intravenous hydrocortisone and a salbutamol nebuliser.
The trial profile is shown in Figure 1. For two patients randomised to the BS/BS group, technical problems were experienced during set-up of the equipment and before introduction of any PW into the GC. These cases have been excluded from the analysis. In all other cases, the wires (as allocated at randomisation) were successfully passed to the desired locations in the target coronary vessels and paired pressure recordings were obtained. The target vessels involved the territories of all three major vessel systems – left anterior descending (LAD) 57%, circumflex (Cx) 21% and right coronary artery (RCA) 22%. Nitrates were administered in all but five cases in which the operator felt that the systemic blood pressure was too low for administration.
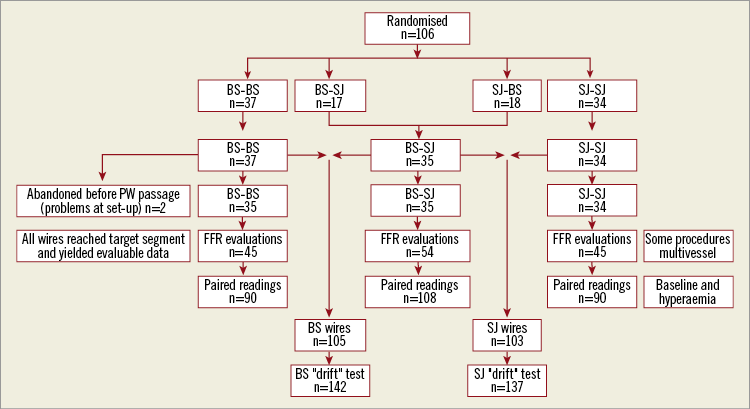
Figure 1. Trial profile. Description of the management of all patients randomised and the number of observations made for each of the analyses in the study.
Combining Pd/Pa and FFR readings, the total number of paired observations for the groups were: BS/BS 90; BS/SJ 108; SJ/SJ 90. Observations on drift were available from 142 vessels for the BS wire and 137 vessels for SJ.
Figure 2 shows the frequency distribution histograms for the difference observed between simultaneous measurements from the randomised wire pairs and the Bland-Altman plots for these data. The observed FFR values are in the range typically found in clinical practice. There is a good level of agreement for all wire pairs. From the Bland-Altman analysis, the 95% levels of agreement were good: BS/BS –0.044 to 0.048; SJ/SJ –0.058 to 0.062; SJ/BS –0.054 to 0.057.
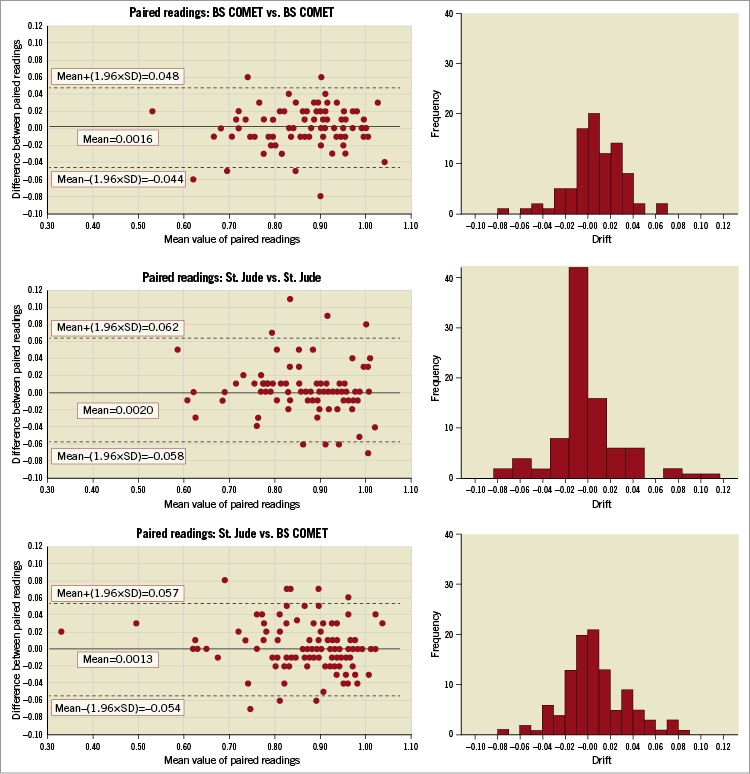
Figure 2. Frequency histogram and Bland-Altman plots for the differences observed in simultaneous readings from the wire pairs, as allocated at randomisation. For each pair of wire types, the magnitude of the differences observed in the simultaneous readings is shown as a frequency histogram. A Bland-Altman plot has been constructed for each wire pair grouping. This plots each observed difference against the mean of the two individual readings and displays the mean of these differences and the associated±two standard deviation values.
The primary analysis considered the differences observed with SJ/BS and SJ/SJ. The mean, (SD), (95% CI for the mean) differences were similar: SJ/BS 0.001, (0.028), (–0.004 to 0.007); SJ/SJ 0.002, (0.03), (–0.004 to 0.008). The median (IQR) values for the absolute difference, irrespective of sign, were also similar: SJ/BS 0.015 (0.01 to 0.03); SJ/SJ 0.01 (0.00 to 0.03), p=0.61.
Figure 3 shows the distribution of observed drift for the two wire types. The mean, (SD), (95% CI for the mean) were similar: BS 0.018, (0.039), (0.012 to 0.025); SJ 0.016, (0.039), (0.011 to 0.021). The drift, expressed as the median (IQR) difference in Pd/Pa from 1.0 (irrespective of sign), was similar: BS=0.02 (0.01-0.05); SJ=0.02 (0.01-0.04); p=0.14.
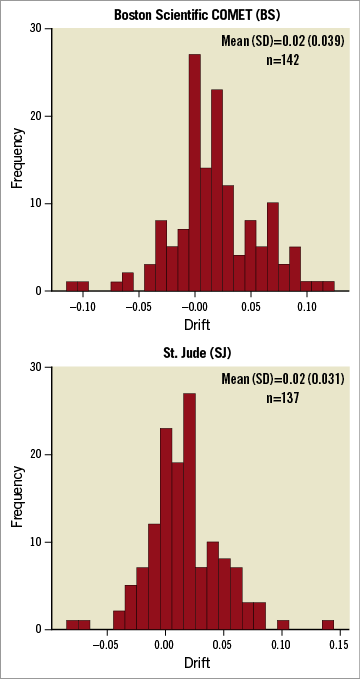
Figure 3. Frequency histograms for the “drift” observed for the two wire types. For each wire type this figure displays, as a frequency histogram, the deviation from unity observed on withdrawal of the wire back into the guide catheter (“drift”).
Discussion
By virtue of its methodology, this study provides robust information about the clinical performance and consistency of pressure recording for the wires tested. The study would suggest that both wires were safe, with no wire-related adverse events. Once introduced into the guide catheter, all wires could be advanced to the desired location for physiological testing in the coronary arteries and pressure recordings were obtained in all cases.
For both systems there appears to be similar consistency in terms of both “within wire type” and “between wire type” performance. The magnitude of variation for the different wire types, in terms of the Bland-Altman 95% limits of agreement, was –0.054 to 0.057. This compares favourably to the same index reported in two studies comparing FFR derived from a microcatheter device and invasive wire assessment (–0.129 to 0.069)9, and (–0.12 to 0.071)10, and also to a study comparing angiographic analysis and invasive wire assessment (–0.096 to 0.112)13 .
There is current interest in the comparison of different approaches to the assessment of the functional significance of coronary disease. These include not only new devices for invasive FFR9,10 but also angiographic methods of FFR determination13 and an examination of alternative indices derived from pressure measurements such as the instantaneous free wave ratio (iFR) and resting Pd/Pa ratio14-16. The novel design of our study includes randomisation between different paired groups of the methods being evaluated. One key feature of the design is that it allows the reported variation between method types to be compared to that which might be expected with repeat use of the same tool. Importantly, our study demonstrated that the magnitude of difference in simultaneous paired observations for the Boston Scientific wire and the St. Jude comparator was no greater than that observed with two identical St. Jude reference wires. The method also allows us to provide information about the apparent reproducibility of the individual wire types and to draw inferences about their clinical utility in terms of the ability of operators to manoeuvre the wires in the coronary vasculature. The absence of wire-related adverse events is reassuring but it must be remembered that the sample size was small and the upper boundary of the 95% confidence interval for a “zero event” point estimate is a function of the number of observations made. This figure would be about 2.7% in this study, as each wire was used in approximately 140 individual epicardial vessels.
Our study also reports important information about “drift”. Although there was no difference between the wires in terms of this measure, the median (IQR) absolute drift observed (irrespective of sign) was 0.02. However, some 25% of the observations demonstrated substantial deviation from the expected unity (BS 0.5, SJ 0.4). These results may have important implications for clinical practice as this unpredictable change in wire calibration could potentially result in incorrect assessment of FFR and consequent inappropriate patient management.
Limitations
Although the study size allows a robust assessment of the magnitude of variation in measured pressure ratios, patient numbers are too small to draw robust conclusions about the incidence of adverse events or about the frequency of failure of a wire to cross a lesion. The protocol did not demand the performance of an additional check of the calibration of the conventional transducer at the time of drift quantification, and hence we cannot exclude the possibility that some element of any imprecision noted may be attributable to factors other than the PW system. We did not record the precise duration of wire use in a specific coronary vessel, and hence cannot report whether the magnitude of observed drift is a function of time from last equalisation. These were routine cases (rather than a specific isolated PW examination), with a typical range of duration and procedural complexity, and hence we believe that the reported drift will mirror that encountered in clinical practice.
Conclusions
We did not detect any difference in the performance of the COMET and St. Jude pressure wire systems in terms of safety or efficacy. The use of both wires was associated with some drift; more work will be required to establish methods for the routine assessment of drift in clinical practice and guidelines on how to respond when variation is identified.
| Impact on daily practice We present a novel, randomised design to test the agreement of measurement between the new COMET pressure wire from Boston Scientific and the St. Jude (Abbott) pressure wire system. We did not detect any difference in the performance of the two wire systems. |
Acknowledgements
University Hospital Southampton. Research staff: Sam Gough, Judy Radmore, Karen Banks, Julie Bigg. Interventional cardiologists: Michael Mahmoudi. Simon Corbett, James Wilkinson, John Rawlins, Alison Calver, Iain Simpson. All catheter laboratory staff, with special thanks to Irvin Verallo, Phil Banks, Sarah Kingston and the cardiac physiology team.
Liverpool Heart and Chest Hospital. Research staff: Jan Barton, Alexandra Thompson. Interventional cardiologists: John Morris, Mike Fisher, Joe Mills. All catheter laboratory staff, with special thanks to Paul Wright, Emer Burton and the cardiac physiology team.
Funding
The study was funded by an unrestricted grant from Boston Scientific Corporation. The funder had no role in the design, performance, analysis or reporting of the study.
Conflict of interest statement
N. Curzen reports grants from Boston Scientific, during the conduct of the study; personal fees from Abbott Vascular, personal fees from Boston Scientific, personal fees from HeartFlow, personal fees from Haemonetics, grants from HeartFlow, grants from Haemonetics, outside the submitted work. R. Stables reports grants from Abbott Vascular, personal fees from Abbott Vascular, personal fees from Philips, personal fees from Boston Scientific, outside the submitted work. Z. Nicholas reports grants from Boston Scientific, during the conduct of the study. The other authors have no conflicts of interest to declare.

