Abstract
Aims: Limited data exist on radial access in carotid artery stenting. This multicentre prospective randomised study was performed to compare the outcome and complication rates of transradial and transfemoral carotid artery stenting.
Methods and results: The clinical and angiographic data of 260 consecutive patients with high risk for carotid endarterectomy, treated between 2010 and 2012 by carotid stenting with cerebral protection, were evaluated. Patients were randomised to transradial (n=130) or transfemoral (n=130) groups and several parameters were evaluated. Primary combined endpoint: major adverse cardiac and cerebral events, rate of access-site complications. Secondary endpoints: angiographic outcome of the procedure, fluoroscopy time and X-ray dose, procedural time, crossover rate to another puncture site and hospitalisation in days. Procedural success was achieved in all 260 patients (100%), the crossover rate was 10% in the TR and 1.5% in the TF group (p<0.05). A major access-site complication was encountered in one patient (0.9%) in the TR group and in one patient (0.8%) in the TF group (p=ns). The incidence of major adverse cardiac and cerebral events was 0.9% in the TR and 0.8% in the TF group (p=ns). Procedure time (1,620 [1,230-2,100] vs. 1,500 [1,080-2,100] sec, p=ns) and fluoroscopy time (540 [411-735] vs. 501 [378-702] sec, p=ns) were not significantly different, but the radiation dose was significantly higher in the TR group (195 [129-274] vs. 148 [102-237] Gy*cm2, p<0.05) by per-protocol analysis. Hospitalisation days were significantly lower in the TR group (1.17±0.40 vs. 1.25±0.45, p<0.05). By intention-to-treat analysis there was a significantly higher radiation dose in the TR group (195 [130-288] vs. 150 [104-241], p<0.05), but no difference in major events (0.9 vs. 0.8, p=ns) and length of hospitalisation in days (1.4±2.6 vs. 1.25±0.45, p=ns).
Conclusions: The transradial approach for carotid artery stenting is safe and efficacious; however, the crossover rate is higher with transradial access. There are no differences in the total procedure duration and fluoroscopy time between the two approaches but the radiation dose is significantly higher in the radial group, and the hospitalisation is shorter with the use of transradial access by per-protocol analysis. By evaluating the patient data according to intention-to-treat analysis we found no difference in major adverse events and hospitalisation. In both groups, vascular complications rarely occurred.
Abbreviations
CAS: carotid artery stenting
CCA: common carotid artery
CEA: carotid endarterectomy
CFA: common femoral artery
CT: computer tomography
DAP: dose area product
ECA: external carotid artery
FA: femoral artery
GW: guidewire
ICA: internal carotid artery
ITT: intention-to-treat
MACCE: major adverse cardiac and cerebral events
MACE: major adverse cardiac events
MRI: magnetic resonance imaging
NASCET: North Atlantic Society of Endovascular Therapy
PAD: peripheral artery disease
PP: per-protocol
RAO: radial artery occlusion
RAS: radial artery spasm
SA: subclavian artery
TB: transbrachial
TC: transcervical
TCD: transcranial Doppler
TF: transfemoral
TR: transradial
Introduction
Carotid artery stenting (CAS) has become an increasingly tenable alternative to carotid endarterectomy (CEA) for occlusive disease in high-risk patients1,2. The concept of CAS is to lower the mortality in surgically high-risk patients and to be an effective treatment alternative. Therefore, avoiding or managing complications becomes crucial in CAS. The results are constantly improving because of the introduction of new embolic protection devices and small calibre stenting systems. The conventional approach to access the common carotid artery (CCA) during endovascular interventions is through the femoral artery (FA); however, this approach is not always feasible because of vessel pathology or aberrant anatomy of the iliofemoral arteries and the aortic arch. The transbrachial (TB)3 and the direct transcervical (TC)4 approaches are alternative access sites for CAS when femoral access is not available. The rationale for the TR approach was to attempt to reduce the incidence of bleeding complications at the vascular access site and to avoid the necessity for prolonged bed rest. Several pilot studies have confirmed the safety and efficacy of transradial CAS5-8 but no randomised prospective trial exists comparing the transfemoral (TF) and transradial (TR) approach for CAS. Our multicentre prospective randomised study is the first to compare the outcome and complication rate between TR and TF access for carotid artery stenting (CAS).
Materials and methods
STUDY POPULATION (Figure 1)
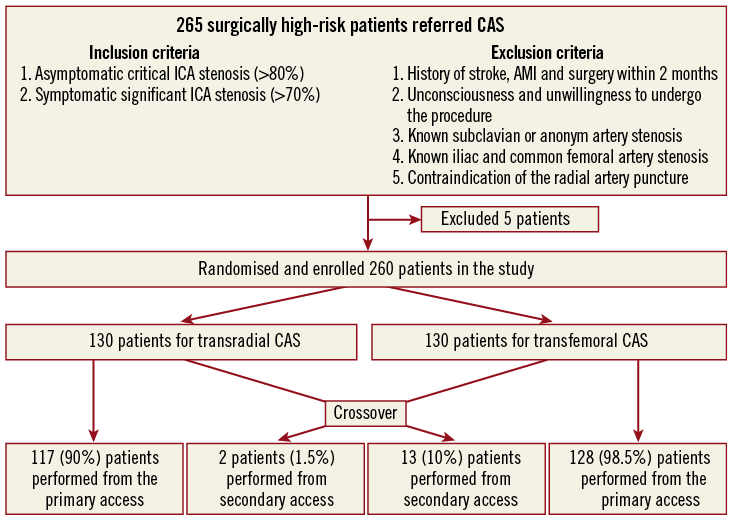
Figure 1. Process of screening, exclusion/inclusion, randomisation and crossover.
The clinical and angiographic outcomes of 265 consecutive patients with high risk for CEA9 treated by CAS with cerebral protection were evaluated in a prospective randomised multicentre study between 2010 and 2012. Five patients were excluded from the study due to unmet angiographic inclusion criteria. One hundred and fifty-eight symptomatic patients with >70% carotid stenosis and 102 asymptomatic patients with >80% stenosis were enrolled. Patients were randomised to TR (n=130) or TF (n=130) groups. Randomisation was performed blindly using previously sealed envelopes. TR cases were performed by three skilled transradial operators. All femoral access sites were closed with a femoral closure device.
ENDPOINTS
The following parameters were applied to evaluate the potential advantages of TR access:
– Primary endpoint: major adverse cardiac and cerebral events (MACCE), rate of major and minor access-site complications.
– Secondary endpoints: angiographic outcome of the CAS, and consumption of the angioplasty equipment, fluoroscopy time and X-ray dose, procedural time, crossover to another puncture site and hospitalisation days.
INCLUSION AND EXCLUSION CRITERIA
Inclusion criteria were: 1) symptomatic (history of stroke or transient ischaemic attack within six months) internal carotid artery stenosis (>70%) determined by magnetic resonance imaging (MRI) or computer tomography (CT), and 2) critical asymptomatic (80%) ICA stenosis by MRI or CT.
Exclusion criteria were: 1) history of acute or recent stroke (<2 months), myocardial infarction, and surgery or trauma within the preceding two months, 2) unconsciousness or unwillingness to undergo the procedure, 3) known subclavian or brachiocephalic artery stenosis, 4) known iliac or common femoral stenosis, and 5) contraindications of the transradial access (negative Allen test, non-palpable radial artery).
ANTITHROMBOTIC REGIMEN
All patients were on dual antiplatelet therapy for two months (aspirin 100 mg and clopidogrel 75 mg per os) started at least three days before the procedure. An intra-arterial cocktail (2.5 mg verapamil, 5,000 IU sodium heparin, 250 mcg nitroglycerine) was given directly in the radial artery through the sheath. Additional Na-heparin was given up to 100 IU/kg.
VASCULAR ACCESS AND PROCEDURE
For the radial approach, an ischaemic Allen’s test contraindicated the procedure. After local anaesthesia (1% lidocaine), the modified Seldinger technique was used for cannulating the radial artery with a 4-5 Fr dedicated transradial sheath (10 cm, 4-5 Fr; Terumo Co., Tokyo, Japan). The sheath was changed for a 6 Fr long kinking resistant guiding sheath or for a 7 Fr short sheath. After the procedure, the sheath was removed immediately and haemostasis was achieved with a tourniquet for six hours. We did not apply a dedicated haemostatic device. Despite radial artery access the patients were immobilised in the intensive care unit for six hours.
For the femoral approach, after local anaesthesia the femoral artery was punctured with a 19-gauge needle through which a J-wire was advanced into the femoral artery. In all cases a 5 Fr short sheath was introduced initially, then the procedure was performed using a 6 Fr 90 cm long sheath. The sheath was removed immediately after the procedure and a closure device (Angio-Seal™; St. Jude Medical, St. Paul, MN, USA) was used. A mechanical compression bandage was used for four hours and the patients were mobilised afterwards.
ANGIOPLASTY PROCEDURE
CAS was performed under local anaesthesia without sedation. Blood pressure, pulse and oxygen saturation were continuously monitored throughout the procedure and neurologic assessment was performed by experienced nursing staff.
Transfemoral CAS was performed according to the standard clinical practice with a guiding sheath, using the Carotid WALLSTENT (Boston Scientific Corporation, Natick, MA, USA), Cristallo Ideale (Medtronic-Invatec, Frauenfeld, Switzerland), and Precise (Cordis Corporation, Bridgewater, NJ, USA) stents. In all procedures we used either the Filter wire (Boston Scientific Corp.) or Emboshield (Abbott Vascular, Santa Clara, CA, USA) cerebral protection device. Post-dilation, to the diameter of the internal carotid artery (ICA), was performed in all patients. Completion angiography was then performed, and a closure device was used to achieve haemostasis in all cases.
TRANSRADIAL CAS
After the sheath insertion (5 Fr; Terumo), diagnostic angiography was performed with a 5 Fr Simmons 1 (Cook Medical, Bloomington, IN, USA) diagnostic catheter with the “pullback and rotate” technique. First, an aortography was performed in LAO 30 projection with small contrast volume (15 ml at 10 ml/s) to visualise the aortic arch, then the non-symptomatic or non-severely stenotic carotid artery was cannulated. Secondly, the diseased common carotid artery (CCA) was deeply engaged with the Simmons 1 catheter (Figure 2, Figure 3). A long J-tip guidewire (GW) was advanced in the external carotid artery (ECA) under “road map” guidance and the CCA was cannulated with a 7 Fr guiding catheter or with a 6 Fr guiding sheath. If the guiding catheter technique was used, the 5 Fr radial sheath was exchanged for a 7 Fr short sheath and a 7 Fr XF 40 (Boston Scientific Corp.) guiding catheter was used for cannulation of the CCA. If the guiding sheath technique was used, a 6 Fr kinking resistant (Cook, Bloomington, IN, USA) guiding sheath was used for the CCA cannulation. In the case of insufficient guiding or sheath support, firstly a buddy wire was used to advance the system forward in the CCA. In failed cases when the guiding catheter or the sheath jumped out in the aortic arch, the telescoping technique was used with the Simmons catheter inside the XF 40 guiding catheter. In case of a failed telescoping attempt, we changed to the loop technique (the guiding catheter was pushed ahead while the guidewire was kept strongly in the operator’s hand creating a loop [loop technique], Figure 4). The stenting was performed the same way as for the femoral CAS, using distal protection.
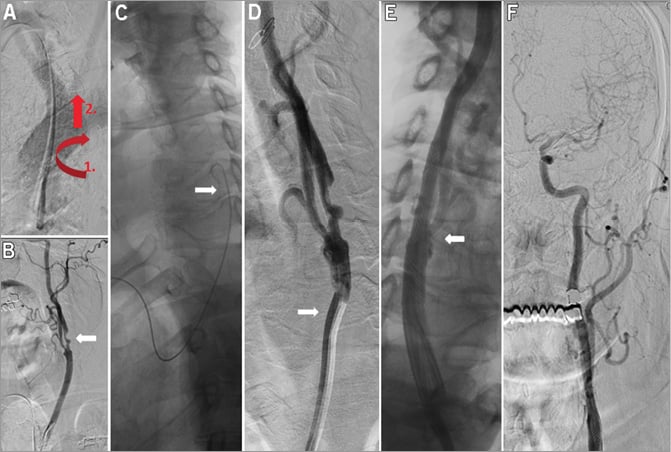
Figure 2. Left ICA stenting in aortic arch type I. Digital substraction image of the aortic arch shows aortic arch type I (A). The Simmons catheter was pulled back with clockwise rotation (pull and rotate technique - red arrows) (A) and the left CCA was cannulated (B). Angiography from the left CCA shows significant ICA stenosis (B, white arrow). The 260 cm long GW is shown in the left CCA (C, white arrow), followed by the cannulation of the left CCA with a 7 Fr XF 40 guiding catheter (D, white arrow). Final angiographies show no residual stenosis after stenting (E & F).
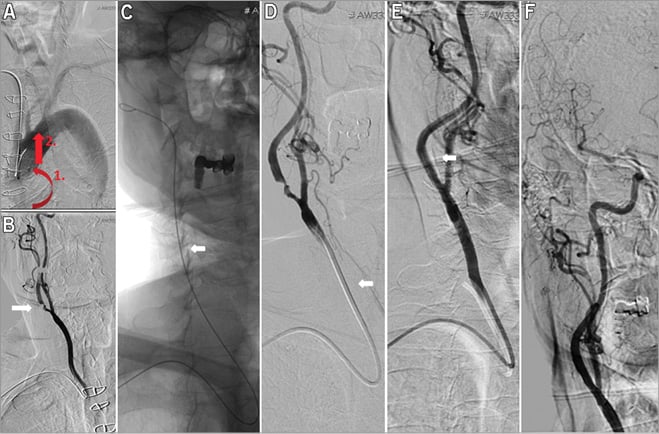
Figure 3. Right ICA stenting in aortic arch type II. Aortography pictures of a 65-year-old patient with right-sided ICA stenosis and aortic arch II. The Simmons catheter was pulled back in the right CCA with anticlockwise rotations (A). Critical stenosis at the origin of the right ICA (B, white arrow). The Starter™ 260 cm long 0.035 GW (Boston Scientific) was advanced deeply in the ECA (C, white arrow) and an XF guiding catheter was advanced in the right CCA (D, white arrow). Angiography after Filter wire advancement and stenting (E, white arrow). Final angiography shows good stent expansion and cerebral flow with patent cerebral arteries (E & F).
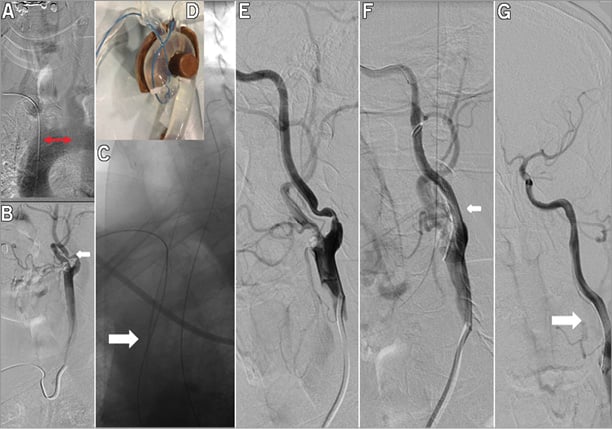
Figure 4. Left ICA stenting in aortic arch type I (loop technique). A 67-year-old patient with level I aorta and left-sided CCA stenosis. Angiography performed with a Simmons I diagnostic catheter revealed a high grade calcified left ICA and ECA lesion (A & B, white arrow). The GW and the XF 40 7 Fr guiding advancement was unsuccessful due to the wide distance between the origin of the left CCA (red arrow). Therefore, the “loop technique” was used with pushing the GW against resistance creating a loop in the aortic arch (C, white arrow). D) shows the loop technique in aortic demonstration model. E) shows the guiding catheter and stent in the left CCA and ICA (white arrow). The left ICA before and after stenting (F & G, white arrow).
ANGIOGRAPHIC ANALYSIS
Carotid angiography was obtained in a routine manner using at least two different projections. The vessels and lesions were analysed by a computerised quantitative analysis system (Siemens Hicor and General Electric) according to previously described and validated edge-detection algorithms using the North Atlantic Society of Endovascular Therapy (NASCET) criteria10. Minimum lumen diameter, reference vessel diameter and percent diameter stenosis were measured before and after angioplasty.
Definitions
CRITERIA FOR A SUCCESSFUL ANGIOPLASTY
A successful angioplasty was defined as no more than 30 percent post-intervention stenosis by NASCET criteria, and an improvement of at least 20 percent in the degree of stenosis10.
ACCESS-SITE BLEEDING
Access-site bleeding was defined as major if associated with a haemoglobin loss of at least 2 mmol/l, administrations of blood transfusions, vascular repair and prolonged hospitalisation, and minor if bleeding at the vascular access site only resulted in haematoma formation and did not require specific therapy.
MAJOR ADVERSE CARDIAC AND CEREBRAL EVENTS (MACCE)
MACCE were assessed as the composite of stroke, death, non-fatal acute myocardial infarction, repeated revascularisation of the target vessel by PTA or vascular operation during the hospital stay and at 30 days.
STROKE
Stroke was defined as an acute neurological event with focal symptoms and signs lasting >24 hrs. Stroke was considered a complication of carotid revascularisation if it occurred within 30 days of the procedure. Independent certified personnel performed baseline and post-procedure neurological assessments on all patients.
Follow-up
Follow-up included a clinical examination during the hospital stay and at one month. All patients without radial artery pulse underwent vascular ultrasound examination.
Statistical analysis
All statistical analysis was performed with the commercially available software STATISTICA version 8.0 (StatSoft Inc., Tulsa, OK, USA). Categorical variables were presented as absolute numbers and percentages, and continuous variables were expressed as mean±standard deviation or median (with interquartile range) as appropriate. The Shapiro-Wilk test was used for testing the distribution of the data. Proportions were compared using the chi-square test or Fisher’s exact test, and continuous variables were compared by t-test or the Mann-Whitney U test as appropriate. Per-protocol (PP) and intention-to-treat (ITT) analysis was performed in the examined population. Probability values lower than 0.05 were considered to be significant.
Results
Two hundred and sixty-five patients were selected for the study, but five patients were excluded because the ICA stenosis severity did not reach the inclusion criteria. Baseline demographic and clinical characteristics of the total study population (n=260) are shown in Table 1. Most of the demographic data did not differ significantly between the two groups.
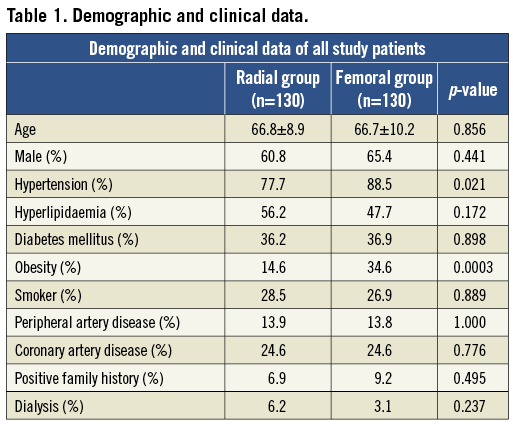
ANGIOGRAPHIC DATA (Table 2)
Angiographic data are summarised in Table 2. Arch type III occurred more frequently in the TR group (p<0.05), but the stenosis location was not different.
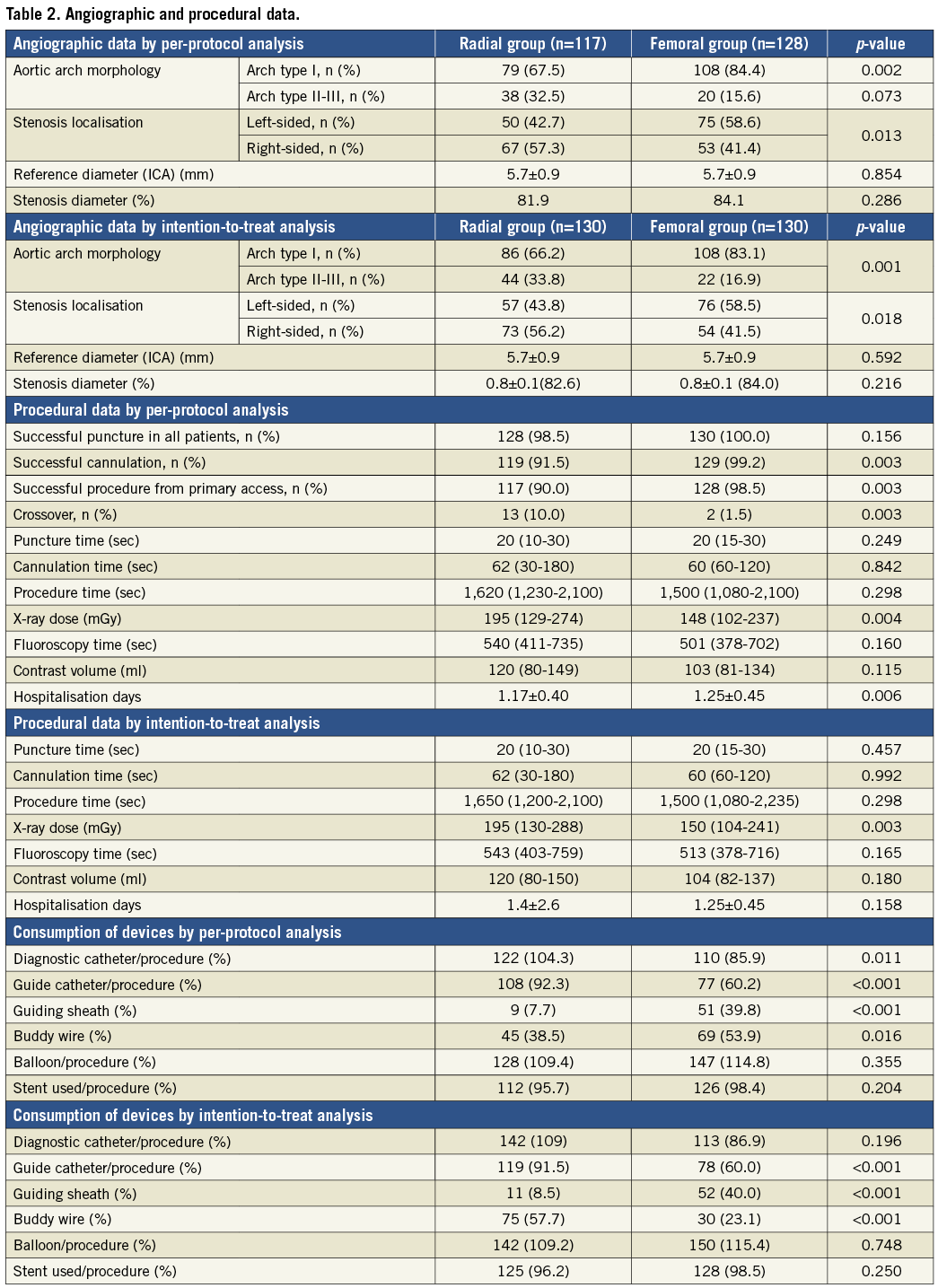
VASCULAR ACCESS AND CROSSOVER (Table 2 and Figure 1)
Successful puncture was achieved in 128 (98.5%) patients in the TR and in 130 patients (100%) in the TF group (p=ns). Procedural success was achieved in all patients (100%). The crossover rate was 10% in the TR group (two failed punctures, one radial artery spasm, one radial artery loop, one subclavian artery stenosis, one subclavian artery tortuosity and six cannulation problems) and 1.5% in the TF group (two iliac artery stenoses) (p<0.05). The cannulation failed in the TR group in six patients (5.12%) due to severe angulation of the right CCA origin from the SA (two/six cases) and to severe angulation of the left CCA (three/six cases) or wide distance of the origin of the left CCA from the innominate artery (one/six cases). All crossover patients were successfully treated from the alternative access. The crossover rate proved to be significantly more frequent from the radial than from the femoral artery (p<0.05). All TR interventions (n=130) were started from the right radial or ulnar artery (129 radial, one ulnar), and TF interventions were performed from the right femoral access in 126 patients (97%) and from the left femoral access in four patients (3%). Crossover examples from the study population are summarised in Figure 5.
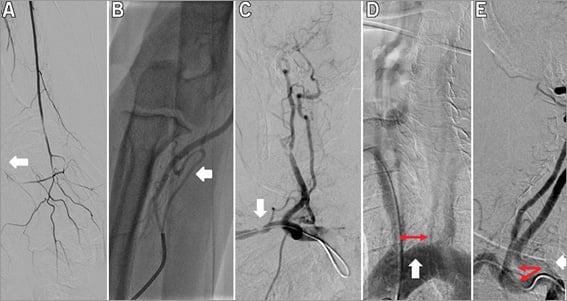
Figure 5. Crossover cases. Radial artery occlusion (A, white arrow), severe radial artery loop (B, white arrow), borderline stenosis in the tortuous SA and sharp angulation between the CCA and the SA (C, white arrow), wide distance between the innominate artery and left CCA (D, red and white arrows), sharp angulation (E, red and white arrows).
CAROTID ANGIOPLASTY PROCEDURAL DATA AND EQUIPMENT (Table 2)
Procedure time (1,620 [1,230-2,100] vs. 1,500 [1,080-2,100] sec, p=ns) and fluoroscopy time (540 [411-735] vs. 501 [378-702] sec, p=ns) were not significantly different, but the radiation dose was significantly higher in the TR group (195 [129-274] vs. 148 [102-237] Gy*cm2, p<0.05) by PP analysis. By ITT analysis there was a significantly higher radiation dose in the TR group (195 [130-288] vs. 150 [104-241], p<0.05), but no difference in major events (0.9 vs. 0.8, p=ns). Table 3 shows the subgroup analysis of the procedural data in different aortic configurations and right and left-sided lesions. The consumption of diagnostic catheters, guiding catheters and buddy wires was significantly higher in the TF group by both PP and ITT analysis. The consumption of balloon catheters and carotid stents proved to be the same for the two groups (Table 2). Hospitalisation days by PP analysis were significantly lower in the TR group (1.17±0.40 vs. 1.25±0.45, p<0.05), but by ITT analysis there was no significant difference between the two groups.
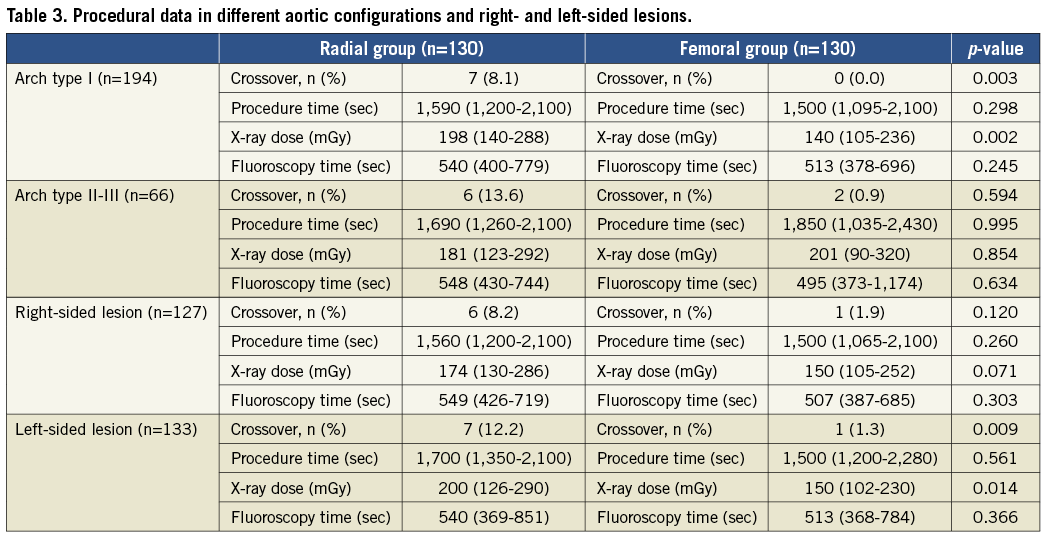
Complications
MAJOR ADVERSE CARDIAC AND CEREBRAL EVENTS (Table 3)
The MACCE rate was 0.9% in the TR and 0.8% in the TF group (p=ns). A transient neurological deficit that resolved completely within 12 hours was noted in 1/128 patients (0.8%) in the femoral group. No periprocedural TIA was observed in the radial artery group, but one patient (0.9%) died as a result of a major stroke 20 days after the procedure. The rate of MACCE in the total population was not different either by PP or by ITT analysis.
RATE OF ACCESS-SITE COMPLICATIONS (Table 4)
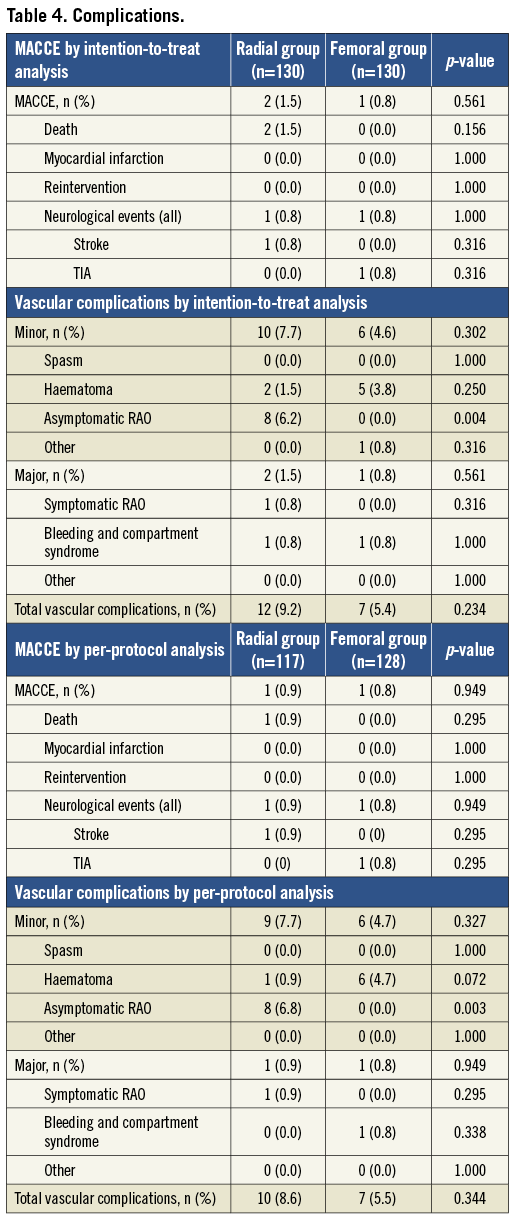
A major access-site complication was encountered in one patient (0.9%) in the TR group (one symptomatic radial artery occlusion in a patient with Buerger’s disease) and in one patient (0.8%) in the TF group (one pseudoaneurysm treated successfully with thrombin injection) (p=ns). Minor access-site complications occurred in nine patients (7%) in the TR and in six patients (4.7%) in the TF group. The cause of minor vascular complications was small forearm haematoma in one patient (0.8%), and asymptomatic RAO in eight patients (6.8%). There was no occurrence of compartment syndrome in the TR group. There were no instances of peripheral nerve injury. In the TF group haematoma occurred in six patients (4.7%). The rates of major and minor vascular complications by PP analysis in the total population including the crossover cases were not significantly different (TF group: 2.3% major and 4.6% minor; TR group: 1.5% major and 7.8% minor) (p=ns).
Discussion
TR access was chosen for CAS for patients with severe peripheral artery disease (PAD), iliac artery tortuosity and in cases of difficult CCA cannulation, as in bovine arch; however, many pilot studies have also confirmed the safety and high success rate of the TB3 and TR5-8 access in a non-selected population.
TRANSFEMORAL ACCESS
TF access is the optimal approach for CAS because the catheter engagement is coaxial and therefore cannulation is easy and safe. Another advantage is that TF access allows the use of all proximal and distal protection systems. Despite this easy access route, the femoral access has many limitations and disadvantages. Significant iliac artery tortuosity and stenosis compromise the responsive movement of GWs and catheters, and the precise implantation of the stents. TF access is also difficult in obese patients and in patients with aortic aneurysm. The rates of major and minor vascular complications are still high after transfemoral CAS11. Another limitation is the prolonged immobilisation after the intervention, resulting in decreased patient comfort. Femoral closure devices were introduced into clinical practice to reduce vascular access complications, and to reduce bed rest, but no significant difference in the rate of vascular complications was observed when compared to manual compression12. Whereas crossing the diseased iliac system has its own risks and challenges, the pathway to the carotid artery via the aortic arch is where the risks begin to include stroke, the very condition the intervention is intended to prevent. Selective cannulation of the arch vessels is technically the most challenging and critical portion of CAS procedures, and it is especially difficult in level II-III aortic arch, bovine arch, tortuous brachiocephalic artery and ostial stenosis. Catheter manipulations in these cases can cause distal plaque disruption and embolisation. Reducing the risk of stroke during CAS requires a combination of proper patient selection and impeccable technique. Transcranial Doppler (TCD) data from multiple studies have shown that there are emboli to the middle cerebral artery during all phases of the procedure13. Faggioli et al found that, during TF, CAS silent cerebral embolisations assessed with diffusion-weighted MRI (DW-MRI) occurred in 57.6% patients both in the contralateral and in the ipsilateral hemisphere (82% contralateral and 18% ipsilateral), suggesting that these embolisations are related not only to the stenting procedure, but also to the cannulation itself14. Difficult cannulation and anatomically high-risk CAS are associated with an increased number of periprocedural strokes15, and the majority of the strokes occur during the cannulation.
TRANSBRACHIAL ACCESS
As an alternative route, brachial and axillary access was introduced at first, but these approaches have been associated with a relatively high risk of vascular and nerve complications3,16.
ADVANTAGES OF RADIAL ACCESS
TR access was first introduced by invasive cardiologists for coronary angiography and angioplasty (PCI), and in many centres this is now the first access line for PCI because of a low vascular complication rate, better patient comfort and immediate mobilisation17-19. The most important advantage of TR access is the low rate of vascular complications, because post-procedural bleeding after acute coronary interventions and in an older population is associated with an increased risk for short- and long-term recurrent bleeding, MACE, and all-cause mortality20. Despite these positive results in acute coronary angioplasty, the association of bleeding complications and MACE was not investigated during TF CAS. Another advantage of TR access during CAS is the easy cannulation of the left CCA in a patient with bovine aorta or in a patient with an elongated left CCA. The right CCA can be easily cannulated from the right radial access as well, but left CCA cannulation might be difficult5-8. By reducing the amount of manipulation required in the aortic arch, the risk of access-related strokes can be reduced. Etxegoien et al6 published the results of the right radial approach for CAS in 382 patients. The crossover rate was 9% and the stroke rate was 0.13% (two major and three minor strokes). Another advantage of the TR technique is increased comfort due to the ability to sit up after the procedure and the fast mobilisation which results in shortened hospitalisation. Shortened hospitalisation was observed by PP analysis in our radial patients; however, by ITT analysis this benefit of the RA access disappeared, because the patients converted to TF were immobilised longer.
DISADVANTAGES AND COMPLICATIONS OF RADIAL ACCESS
Several complications were reported after TR intervention, including radial artery spasm (RAS), occlusion (RAO), perforation and compartment syndrome, pseudoaneurysm formation, and distal embolisation21,22. RAS is a common problem and in most cases can be prevented with intra-arterial vasodilators and/or hydrophilic guidewires. In our patient population, severe RAS was responsible for crossover in only one case despite sheath upsizing for a 6-7 Fr sheath. Forearm compartment syndrome followed by radial artery perforation can be a serious complication; however, immediate diagnosis and forearm bandage can prevent the occurrence of compartment syndrome in the vast majority of cases21,22. In our study population, only one perforation occurred, and it was treated successfully with arm bandage. RAO is a frequent (1-3%) complication after transradial interventions and has no clinical importance if the palmar arch is complete23. Asymptomatic RAO occurred in 10% of cases when a large (7-8 Fr) sheath was used in a small series24,25; however, in our study population RAO occurred in only eight patients (6.15%) and only one patient was symptomatic. Uhlemann et al reported a significant increase in RAO when 6 Fr was used (RAO at 5 Fr=14.4% and at 6 Fr=33.1%, p<0.01)25 compared with 5 Fr, and symptomatic RAO occurred in 7% of the cases. Of patients with RAO, 59% were treated with low molecular weight heparin (LMWH). The recanalisation rates were significantly higher in patients receiving LMWH compared with conventional therapy (55.6% vs. 13.5%, p<0.001) after a mean of 14 days. RAO is asymptomatic in most cases, but there are case reports of symptomatic RAO and successful radial artery recanalisations26. Despite the low incidence of RAO, the radial artery must be preserved for further interventions, and in some cases for RA harvest and Cimino fistula surgery. For this reason, all preventive measures must be taken to prevent RAO, including fast and atraumatic puncture, intra-arterial administration of heparin and verapamil, and the use of a non-occlusive bandage27.
An important disadvantage is the increased radiation exposure during TR access. In our patient population, the radiation dose was 29% higher in the TR group by PP and 27% by ITT analysis. Mercuri et al28 found that transradial coronary angiography was associated with increased radiation exposure to the patient (23% increase in DAP) when compared with femoral access, but the main criticism of the study was that the centre was not primarily a TR centre. Operator volume and learning curve issues are also powerful mediators of radiation exposure29.
Limitations of transradial access
A limitation of TR access is that the puncture of the RA is sometimes difficult due to the small size of the artery. Another anatomical limitation is the RA loop or tortuosity which makes catheter advancement difficult. In difficult J-tip guidewire advancement, an early selective angiography and the use of a hydrophilic Terumo guidewire (Terumo Co.) solved the problem in most cases in our population. The main limitation of the technique is the difficult cannulation of the CCA which was related in right-sided lesions to the sharp angulation of the bifurcation between the CCA and the right subclavian artery and in left-sided lesions to type I and III aorta. In these situations, the use of a buddy wire and the use of the loop technique solved the problem in most cases. The loop technique failed when the 90 cm long sheath did not reach the CCA ostium and the aortic valve was not strong enough and therefore the sheath jumped in the left ventricle. In these cases, the TR technique was not forced and the procedure was finalised from a TF approach. In all cases using the loop technique, a “cough test” was performed before the intervention.
Study limitations
An important limitation of the study is that microembolisation was not assessed with TCD and diffusion-weighted MRI. Also, the cognitive test was not performed after the procedure. The fairly low number of the patient population may also limit our conclusions.
Take-home message
Radial artery access is recommended for patients with high bleeding risk (obese patients, concomitant peripheral artery disease) and for patients with difficult common carotid artery cannulation (aortoiliac disease or severe tortuosity, bovine arch and type II-III aortic arch). Femoral access should be addressed in patients with type I aortic arch and good femoral access.
Conclusions
The transradial approach for carotid artery stenting is safe and efficacious; however, the crossover rate is higher with transradial access. There are no differences in the total procedure duration and fluoroscopy time between the two approaches, but the radiation dose is significantly higher in the radial group and the hospitalisation is shorter with the use of transradial access by per-protocol analysis. By evaluating the patient data according to intention-to-treat analysis, we found no difference in major adverse events and hospitalisation. In both groups, vascular complications occurred rarely.
| Impact on daily practice We recommend the anatomy-related individualised access strategy for CAS after visualisation of the supra-aortic vessels with CT angiography. In type II-III aorta and in patients with severe PAD the radial access site is preferred but, in patients with type I aorta, the femoral access site is preferred. Despite these promising results, the authors suggest beginners in transradial technique should first start with diagnostic procedures to be familiar with the puncture and crossing the supra-aortic vessels, and should learn the proper cannulation of the CCA with the Simmons catheter. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

