Abstract
The optimal antiplatelet strategy after coronary artery bypass graft (CABG) surgery in patients with chronic coronary syndromes (CCS) is unclear. Adding the P2Y12 inhibitor, ticagrelor, to low-dose aspirin for 1 year is associated with a reduction in graft failure, particularly saphenous vein grafts, at the expense of an increased risk of clinically important bleeding. As the risk of thrombotic graft failure and ischaemic events is highest early after CABG surgery, a better risk-to-benefit profile may be attained with short-term dual antiplatelet therapy followed by single antiplatelet therapy. The One Month Dual Antiplatelet Therapy With Ticagrelor in Coronary Artery Bypass Graft Patients (ODIN) trial is a prospective, randomised, double-blind, placebo-controlled, international, multicentre study of 700 subjects that will evaluate the effect of short-term dual antiplatelet therapy with ticagrelor plus low-dose aspirin after CABG in patients with CCS. Patients will be randomised 1:1 to ticagrelor 90 mg twice daily or matching placebo, in addition to aspirin 75-150 mg once daily for 1 month; after the first month, antiplatelet therapy will be continued with aspirin alone. The primary endpoint is a hierarchical composite of all-cause death, stroke, myocardial infarction, revascularisation and graft failure at 1 year. The key secondary endpoint is a hierarchical composite of all-cause death, stroke, myocardial infarction, Bleeding Academic Research Consortium (BARC) type 3 bleeding, revascularisation and graft failure at 1 year (net clinical benefit). ODIN will report whether the addition of ticagrelor to low-dose aspirin for 1 month after CABG reduces ischaemic events and provides a net clinical benefit in patients with CCS. (ClinicalTrials.gov: NCT05997693)
The majority of patients undergoing coronary artery bypass graft (CABG) surgery present with chronic coronary syndromes (CCS)1. Saphenous vein grafts (SVG) are used in approximately 90% of CABG procedures2. Aspirin (acetyl salicylic acid [ASA]) is the standard of care after CABG to reduce SVG occlusion and adverse cardiovascular events. Although dual antiplatelet therapy (DAPT) with a P2Y12 inhibitor in addition to ASA is recommended in patients undergoing CABG for acute coronary syndromes (ACS)3, the role of DAPT in patients with CCS undergoing CABG is unclear.
In an individual patient-data meta-analysis of all randomised clinical trials (RCTs) comparing ticagrelor DAPT with ASA after CABG, ticagrelor DAPT − with a median treatment duration of 12 months − was associated with a significantly lower incidence of SVG failure (11.2% vs 20%; odds ratio [OR] 0.51, 95% confidence interval [CI]: 0.35-0.74; p<0.001), a finding that was consistent across subgroups, including patients with CCS4. Ticagrelor DAPT was also associated with a significant reduction of the composite of SVG failure or cardiovascular death (13.9% vs 23.4%, OR 0.52, 95% CI: 0.36-0.76; p<0.001). However, these benefits were accompanied by a significantly increased risk of clinically important bleeding events (defined as Bleeding Academic Research Consortium5 [BARC] type 2, 3, or 5): 22.1% for ticagrelor DAPT versus 8.7% for ASA (OR 2.98, 95% CI: 1.99-4.47; p<0.001). These findings underscore the need for post-CABG antiplatelet regimens that reduce bleeding risk while preserving efficacy against ischaemic events.
Thrombosis is the predominant mechanism of early SVG failure and typically occurs during the first month after surgery67. Graft thrombosis is driven by platelet activation and aggregation8. The pathophysiology of SVG failure provides a biological rationale for intensified antiplatelet therapy in the first month after CABG. In fact, despite the use of ASA, approximately 10-15% of SVG fail early after surgery7, and graft failure is associated with adverse cardiac events and death910. Data from contemporary CABG trials show that the rate of ischaemic events is highest in the first month after CABG and decreases markedly thereafter, remaining at a constantly lower rate for up to 5 years after surgery1112131415. A short-term intensified antiplatelet regimen therefore seems biologically and clinically justified.
In the PEGASUS and THEMIS trials which tested ticagrelor DAPT versus aspirin alone in patients with CCS and high-risk coronary artery disease, bleeding events accrued at a near-constant rate during follow-up, and the excess bleeding risk with ticagrelor DAPT remained stable over time with a hazard ratio >2.0161718. This suggests that the increased risk of bleeding with ticagrelor DAPT is related to the duration of treatment, and when DAPT is used for ≥12 months, the excess bleeding risk may partially offset the ischaemic benefit. This further strengthens the rationale for a short-term, intensified antiplatelet regimen to balance the ischaemic benefit with the bleeding risk.
Several recent RCTs have shown the benefit of a shorter duration of DAPT in stable patients after percutaneous coronary intervention (PCI) with drug-eluting stents avoiding exposing patients to long-term bleeding risks19. A short-term intensified antiplatelet regimen after CABG is consistent with contemporary PCI practice of limiting the duration of DAPT to the postprocedural period during which endothelialisation of the stent occurs.
Methods
ODIN TRIAL DESIGN
STUDY OBJECTIVES
The primary objective of the One Month Dual Antiplatelet Therapy With Ticagrelor in Coronary Artery Bypass Graft Patients (ODIN) trial is to compare the effects of treatment with ticagrelor versus with placebo, in addition to low-dose ASA for 1 month, on the 1-year incidence of ischaemic events and graft failure among patients with CCS undergoing CABG. The secondary objective is to determine the net clinical benefit of short-term ticagrelor versus placebo, in addition to low-dose ASA.
STUDY DESIGN
The ODIN trial (ClinicalTrials.gov: NCT05997693) is an investigator-initiated, prospective, randomised, double-blind, placebo-controlled, international, multicentre trial of 700 subjects at approximately 20 study centres in 6 countries. Eligible patients will be enrolled before CABG and randomised (1:1) after surgery to receive ticagrelor 90 mg twice daily or placebo, in addition to low-dose ASA for 1 month. Follow-up for all randomised subjects includes the assessment of graft status by coronary computed tomography angiography (CCTA) at 1 year, and follow-up will continue for 5 years with an option for additional follow-up for up to 10 years. The study design is shown in Figure 1.
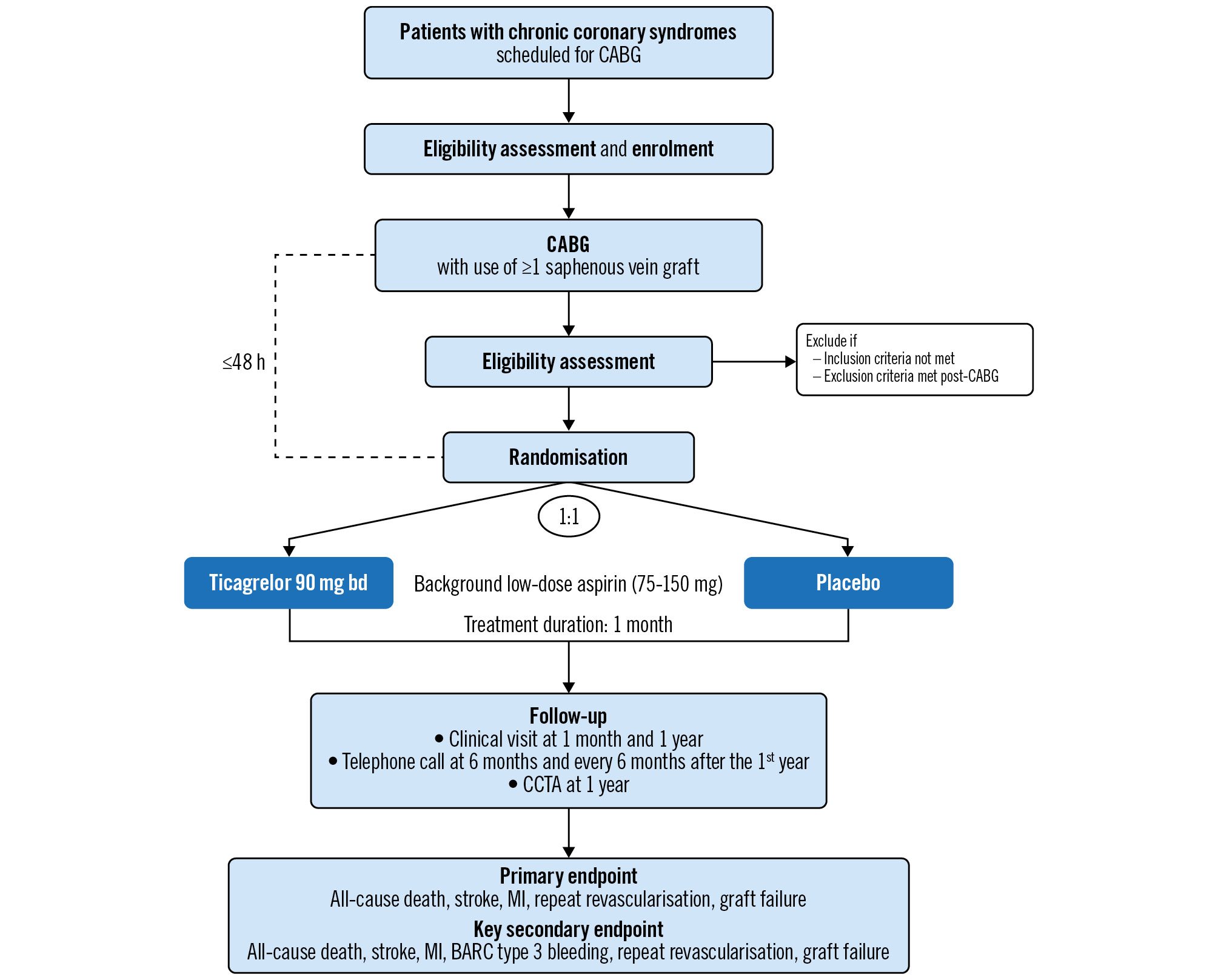
Figure 1. Study flowchart. BARC: Bleeding Academic Research Consortium; bd: twice daily; CABG: coronary artery bypass grafting; CCTA: coronary computed tomography angiography; MI: myocardial infarction
ENROLMENT AND RANDOMISATION
The inclusion and exclusion criteria are shown in Table 1. Patients will be screened for inclusion and enrolled before CABG but will be randomised after surgery to ensure that all eligibility criteria are met. Randomisation will be performed within 48 hours of CABG, when − based on the surgeon’s evaluation − there is minimal bleeding risk. Randomisation after surgery will increase the likelihood that patients receive the randomised treatment and reduce the risk of protocol violation due to perioperative complications. Randomisation after CABG also allows for assessment of heterogeneity of the treatment effect according to how soon after CABG ticagrelor can be initiated. Eligible patients will be randomised (1:1) to ticagrelor or placebo through a centrally controlled, automated web system using permuted block randomisation stratified by study centre and the number of grafts (2 vs ≥3 grafts).
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Age ≥18 years | 1. Any indication for dual antiplatelet therapy, including |
| 2. Elective first-time CABG with use of ≥1 saphenous vein graft | • acute/recent (within 1 year) ACS (NSTE-ACS or STEMI) |
| 3. Ability to sign informed consent and comply with all study procedures, including follow-up for at least 5 years | • recent PCI requiring continuation of dual antiplatelet therapy after CABG |
| 2. Current or anticipated use of oral anticoagulation | |
| 3. Paroxysmal, persistent or permanent atrial fibrillation | |
| 4. Any concomitant cardiac or non-cardiac procedure | |
| 5. Planned cardiac or non-cardiac surgery within 1 year | |
| 6. Preoperative end-organ dysfunction (dialysis, moderate to severe liver failure, respiratory failure), cancer or other non-cardiac comorbidity with a life expectancy <5 years | |
| 7. Inability to use the saphenous vein | |
| 8. Contraindications to the use of aspirin | |
| 9. Contraindications to the use of ticagrelor, including | |
| • known hypersensitivity to ticagrelor | |
| • active pathological bleeding (including but not limited to gastrointestinal or intracranial bleeding) | |
| • history of intracranial haemorrhage | |
| • concomitant therapy with strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, nefazodone, ritonavir, atazanavir) | |
| 10. Inability to undergo CCTA | |
| 11. Participation in another investigational device or drug study | |
| 12. Women of childbearing potential | |
| 13. Any major perioperative complication occurring between CABG and randomisation, including, but not limited to, stroke, TIA, MI, CABG-related bleeding (BARC type 4), sepsis | |
| ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CABG: coronary artery bypass graft; CCTA: computed coronary tomography angiography; MI: myocardial infarction; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischaemic attack | |
TREATMENT PROTOCOL
The ticagrelor dose (90 mg twice daily) is the dose that has been evaluated in prior RCTs of ticagrelor after CABG411. The efficacy and safety of the 90 mg twice-daily dose was established in the PLATelet Inhibition and Patient Outcomes (PLATO) Study20, including the subgroup of patients undergoing CABG21. The initial dose will be administered after randomisation, and a loading dose is recommended. Subsequent maintenance doses will consist of one tablet of active ticagrelor or matching placebo twice daily. The duration of treatment with the study medication for an individual patient will be 1 month. Patients will receive a drug diary in which each study drug dose will be recorded to assess compliance.
All patients will take background open-label low-dose (75-150 mg once daily) ASA administered ideally within 6 hours (and no later than 24 hours) after CABG, consistent with guideline recommendations and dosing labels for ticagrelor.
The use of additional antithrombotic therapy, including other P2Y12 receptor inhibitors and oral anticoagulants, will not be allowed prior to randomisation or for the duration of treatment with the study medication.
All patients will receive secondary preventive measures, including lifestyle modifications and pharmacotherapy, consistent with guideline recommendations2223.
FOLLOW-UP
Randomised patients will return for study visits at 1 month (+14 days) and 1 year (+60 days) (Figure 1). Telephone calls are scheduled at 6 months, and at 6-month intervals after the first year for 5 years. Follow-up may be continued annually for up to 10 years, and patients are preconsented for this option. At each follow-up visit, patients will be assessed for adverse events and potential endpoint events. All patients will undergo CCTA at 1 year for the assessment of graft status by blinded readers, based on Society of Cardiovascular Computed Tomography (SCCT) guidelines24. To protect against inflation of revascularisation rates, clinical sites will remain blinded to data. Details of the CCTA analysis are provided in Supplementary Appendix 1.
ENDPOINTS
The primary endpoint of the trial is the 1-year hierarchical composite of all-cause death, stroke, myocardial infarction (MI), repeat revascularisation and any graft failure (Table 2). The primary endpoint addresses the question of whether 1-month ticagrelor DAPT reduces the risk of ischaemic events and graft failure in the first year after CABG. The key secondary endpoint is the 1-year hierarchical composite of all-cause death, stroke, MI, BARC type 3 bleeding, repeat revascularisation and any graft failure. The key secondary endpoint includes a safety endpoint (BARC major bleeding) and provides an estimate for the 1-year net clinical benefit of 1-month ticagrelor DAPT (Table 2). The hierarchical nature of the composite endpoints accounts for the different clinical priority of the individual endpoint components. Two powered secondary endpoints are prespecified and will evaluate the 5-year effects of 1-month ticagrelor DAPT on clinically important endpoints, quality of life (QoL), and the 5-year net clinical benefit. Endpoints are listed in Table 2. The definitions of the primary and secondary endpoints are shown in Supplementary Table 1.
Table 2. Endpoints.
| Primary and key secondary endpoints (assessed at 1-year follow-up) |
|---|
| Primary endpoint |
| • Hierarchical composite of all-cause death, stroke, MI, repeat revascularisation and any graft failure |
| Key secondary endpoint |
| • Hierarchical composite of all-cause death, stroke, MI, BARC type 3 bleeding, repeat revascularisation and any graft failure |
| Secondary endpoints (assessed at 5-year follow-up) |
| 1. Hierarchical composite of all-cause death, stroke, MI, repeat revascularisation, and 5-year time-averageda disease-specific QoL score |
| 2. Hierarchical composite of all-cause death, stroke, MI, BARC type 3 bleeding, repeat revascularisation, and 5-year time-averageda disease-specific QoL score |
| Exploratory endpoints |
| Assessed at 1 month: |
| • BARC ≥type 2 bleeding |
| • BARC ≥type 3 bleeding |
| • Generic and disease-specific QoL |
| Assessed at 1 year: |
| • Any graft failure (patient and graft level) |
| • Any vein graft failure (patient and graft level) |
| • Any arterial graft failure (patient and graft level) |
| Assessed at 1 year and then annually for 5 years: |
| • Composite of death, stroke, MI or repeat revascularisation |
| •Composite of death, stroke, MI, BARC type 3 bleeding or repeat revascularisation |
| • All-cause death |
| • Cardiovascular death |
| • Stroke |
| • MI |
| • Repeat revascularisation |
| • BARC ≥type 2 bleeding |
| • BARC ≥type 3 bleeding |
| • Generic and disease-specific QoL |
| a Defined as time-averaged QoL over 5 years (or the shared duration of follow-up if follow-up for either patient is <5 years in a given pairwise comparison), based on QoL assessments at 1, 6, 12, 24, 36, 48, and 60 months. All endpoints are adjudicated by an independent clinical events adjudication committee. BARC: Bleeding Academic Research Consortium; MI: myocardial infarction; QoL: quality of life |
QUALITY OF LIFE AND HEALTH ECONOMICS
QoL will be evaluated using both disease-specific and generic instruments. The 7-item Seattle Angina Questionnaire (SAQ) is a validated disease-specific instrument for assessing the health status of patients with coronary artery disease and is a component of the patient-centric powered 5-year secondary endpoints2526. Generic health status using the Medical Outcomes Study 12-item Short Form (SF-12) will also be assessed over the course of follow-up. These measures will be assessed at baseline (prior to randomisation), 1 month, 6 months, 1 year, and annually thereafter until 5 years after surgery.
An economic analysis will be performed from a US perspective. If the intervention is cost-saving or cost neutral, no additional analysis will be performed27. However, if the mean cost per patient in the intervention group exceeds that in the placebo group, a cost-effectiveness analysis will be performed using in-trial survival data and SF-12 scores mapped to EuroQol-5D utility values28 and expressed as cost per quality-adjusted life years.
STATISTICAL CONSIDERATIONS AND SAMPLE SIZE
The main analysis for the primary and secondary endpoints will be performed in the intention-to-treat (ITT) population, defined as all randomised subjects, irrespective of protocol adherence or the duration of exposure to study treatment. Sensitivity analyses will be performed in the modified ITT population, defined as the subset of the ITT population that received at least one dose of randomly assigned study medication.
The primary, key secondary and 5-year secondary endpoints are hierarchical composite endpoints and will each be compared between groups using the win ratio method29 and the joint rank test proposed by Finkelstein and Schoenfeld. The prespecified hierarchies for each endpoint are shown in Supplementary Table 2. Sequential endpoint testing of the primary, key secondary and 5-year secondary endpoints will be employed to preserve type I error.
The primary and key secondary endpoints will also be analysed using Finkelstein-Schoenfeld statistics in the following prespecified subgroups: age (≥ vs <65 years), sex, diabetes mellitus, left ventricular ejection fraction (< vs ≥50%), SYNTAX score (≥ vs <32), complete versus incomplete revascularisation, number of vein grafts, number of arterial grafts, total number of grafts, off- versus on-pump CABG, SVG harvesting technique (endoscopic vs open), bleeding risk (Academic Research Consortium for High Bleeding Risk criteria30 and PRECISE-DAPT score), and gender (according to self-identified gender category at randomisation and gender score [≥ vs Event rate assumptions for endpoint components were based on event rates in contemporary CABG RCTs and an individual patient-data meta-analysis of RCTs investigating the association of ticagrelor DAPT with SVG failure (Supplementary Table 3). With a sample size of 700 patients, the trial has 87.6%, 83.4%, 90.0% and 86.1% power to show superiority of ticagrelor versus placebo for the primary endpoint, key secondary endpoint, and 5-year secondary endpoints, assuming a yearly combined rate of all-cause death, stroke, MI and repeat revascularisation of 7.0% in the placebo group and 5.6% in the ticagrelor group (corresponding to a relative risk reduction [RRR] of 20%); an incidence of graft failure at 1 year of 24.8% in the placebo group and 14.3% in the ticagrelor group (corresponding to an RRR of 42%); 1-month BARC type 3 bleeding rates of 1.2% in the placebo group and 1.5% in the ticagrelor group (corresponding to a relative risk increase of 25%), and a BARC type 3 bleeding rate of 0.7% in both groups between 1 month and 1 year; a difference in the time-averaged SAQ-7 overall summary score of 5 points; loss to follow-up of 2.5% per year; missing data on graft status at 1 year for 7.5% of event-free patients (i.e., patients without death, stroke, MI or repeat revascularisation within 1 year); and a 2-sided significance level of 0.05. A decision on increasing the sample size will be made based on a blinded interim review of aggregate data for the primary endpoint after approximately 80% (n=560) of subjects have been enrolled. The study is coordinated by the principal investigators and the Weill Cornell Medicine Joint Clinical Trials Office. An independent data safety monitoring board will monitor the trial. The ODIN trial will be funded by the Canadian Institutes of Health Research from 2023 to 2030. The primary and key secondary endpoints, as well as exploratory endpoints at 1 month and 1 year, will be reported in a primary publication. The powered 5-year secondary endpoints, as well as exploratory endpoints up to 5 years, will be reported in a subsequent publication. ODIN will report whether the addition of ticagrelor to low-dose ASA for 1 month after CABG reduces ischaemic events and provides a net clinical benefit in patients with CCS. D.J. Angiolillo has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova (now Alta Biomaterials), CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. D.L. Bhatt has had advisory board roles with ANGIOWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier, High Enroll, Janssen, Level Ex, McKinsey & Company, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; he has held board of directors’ positions with ANGIOWave (stock options), Boston VA Research Institute, Bristol-Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, and TobeSoft; he has held Chair roles including inaugural Chair for American Heart Association Quality Oversight Committee; he has held consultant roles with Broadview Ventures, and Hims; and has been on data monitoring committees, including for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research, Boston Scientific, Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, Population Health Research Institute, and Rutgers University; and has received honoraria from various organisations; he has also held other roles with Clinical Cardiology, NCDR-ACTION Registry Steering Committee, and VA CART Research and Publications Committee; he has held a patent for Sotagliflozin; he has received research funding from numerous entities, including AstraZeneca; he has received royalties from Elsevier; he has been a site co-investigator for multiple companies; he has been a trustee for the American College of Cardiology; and he has carried out unfunded research with FlowCo and Takeda. S.E. Fremes has received grant support from CIHR, NIH, Medtronic, Boston Scientific, and Amgen. S.V. Rao has received research funding from NHLBI. J.A. Spertus has provided consultancy services to Alnylam, AstraZeneca, Bayer, Merck, Janssen, Bristol-Myers Squibb, Edwards Lifesciences, Kineksia, 4D Medical, Terumo, Cytokinetics, Imbria, and UnitedHealthcare; he has received research grants from Bristol-Myers Squibb, Abbott, and Janssen; he has held ownership of copyrights to the Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Peripheral Artery Questionnaire; and he has been on the board of directors for Blue Cross Blue Shield of Kansas City. The other authors have no conflicts of interest to declare. To read the full content of this article, please download the PDF.SAMPLE SIZE
TRIAL ORGANISATION AND FUNDING
REPORTING OF TRIAL RESULTS
Conclusions
Conflict of interest statement
Supplementary data

