
Abstract
Heavily calcified lesions may be difficult to dilate adequately with conventional balloons and stents, which causes frequent periprocedural complications and higher rates of target lesion revascularisation (TLR). High-pressure non-compliant balloon angioplasty may be of insufficient force to modify calcium and, even when successful, may be limited in its ability to modify the entire calcified lesion. Scoring and cutting balloons hold theoretical value but data to support their efficacy are lacking and, because of their high lesion crossing profile, they often fail to reach the target lesion. Rotational and orbital atherectomy target superficial calcium; however, deep calcium, which may still impact on vessel expansion and luminal gain, is not affected. Intravascular lithotripsy (IVL), based on lithotripsy for renal calculi, is a new technology which uses sonic pressure waves to disrupt calcium with minimal impact to soft tissue. Energy is delivered via a balloon catheter, analogous to contemporary balloon catheters, with transmission through diluted ionic contrast in a semi-compliant balloon inflated at low pressure with sufficient diameter to achieve contact with the vessel wall. With coronary and peripheral balloons approved in Europe, peripheral balloons approved in the USA and multiple new trials beginning, we review the indications for these recently introduced devices, summarise the clinical outcomes of the available trials and describe the design of ongoing studies.
Introduction
As the population ages and the prevalence of diabetes and renal disease continues to rise, the prevalence of cardiovascular calcium has also risen1,2. Severely calcified lesions remain a challenge for interventional cardiologists. Coronary artery calcification (CAC) impacts on interventional outcomes by impairing stent crossing3, delaminating the drug-eluting polymer from the stent4, affecting drug delivery and elution kinetics, and reducing stent expansion and apposition5. Similarly, calcified peripheral arterial disease (PAD) adds to lesion complexity and is present in 20% of revascularisation procedures6. Transfemoral access for large-bore procedures such as transcatheter aortic valve implantation (TAVI) has become well established as the access of choice7,8,9. Calcified iliofemoral disease is the predominant reason why alternative access sites are utilised10.
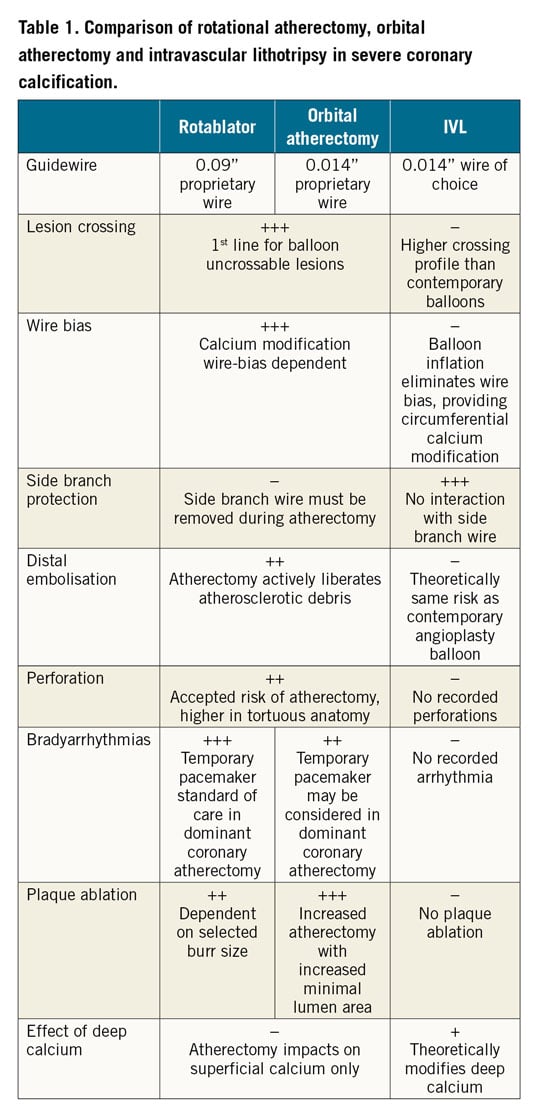
Current techniques to treat calcified lesions including balloon angioplasty, specialty balloons (e.g., cutting, scoring, ultra-high-pressure) and atherectomy have limitations. Balloon dilation, including by specialty balloons, may be of insufficient force to lead to calcium fracture and vessel expansion. Rotational and orbital atherectomy may be biased by the guidewire towards non-calcified segments of the artery11, resulting in ineffective ablation. Even when effective in facilitating stent delivery, atherectomy may have a limited effect on deep calcium, which restricts complete stent expansion and vessel wall apposition5,12. In addition, periprocedural PCI complications including slow flow and periprocedural myocardial infarction (MI) are still significantly higher with atherectomy than traditional balloon-based therapies (Table 1),13,14,15. Calcification also increases the risk of vascular complications following treatments for PAD including dissection, perforation, distal embolisation and restenosis after percutaneous transluminal angioplasty (PTA) or stenting16,17,18.
Intravascular lithotripsy (IVL) is a new technology that delivers pulsatile sonic pressure waves converted to mechanical energy to modify vascular calcium. It leverages similar principles to urologic lithotripsy, which has been used as a safe and effective treatment for kidney stones for several decades19,20. Both therapies use electrohydraulically generated sonic pressure waves that pass through soft tissue, but interact strongly with high-density calcium, producing significant shear stresses that can fracture calcium. In contrast to focal, high-energy urological lithotripsy, the acoustic energy produced by IVL is tuned specifically for vascular applications. IVL safely selects and effectively modifies both intimal and medial calcium21 without negatively impacting on soft tissue across a wide range of vascular applications to increase vessel compliance, and thereby enables the optimal treatment option for the patient and simplifies complex calcified vascular procedures.
With additional peripheral IVL catheters and modifications to the above-the-knee catheters recently cleared for use in Europe and the USA, next-generation coronary IVL catheter approval in Europe, along with multiple clinical trials underway, we provide a timely review on IVL in both coronary and peripheral applications.
Calcific coronary stenoses
DEFINITION AND PROGNOSTIC IMPLICATIONS
The most commonly accepted definition of angiographically severe coronary calcium is the presence of fixed radiopacities, detected without cardiac motion before contrast injection, compromising both sides of the arterial lumen22. While angiography alone underestimates calcium and does not easily allow its quantification23, multidetector coronary computed tomography (CT), a non-invasive technique able to measure calcium score and assess prognosis, is considered by many to be the technique with the greatest diagnostic utility24,25. In the catheterisation laboratory, intravascular imaging techniques can quantify the circumferential and longitudinal distribution of calcium better, and distinguish superficial from deep deposits, thus helping to define thresholds for severity of calcium that would require lesion preparation different from conventional predilatation26,27. With intravascular ultrasound (IVUS), calcium is apparent as bright hyperechoic lines with acoustic shadowing. The optical coherence tomography (OCT) features of calcium are low signal attenuation areas surrounded by well delineated external contours, a very different pattern from lipid deposits that cause high attenuation and preclude detection of the outer border28. An advantage of OCT over IVUS is its ability to measure calcium thickness and detail protruding calcium masses because of its higher resolution. It has recently been described that a calcium length greater than 5 mm, a thickness of greater than 0.5 mm, and an arc greater than 180 degrees are associated with a higher risk of stent underexpansion26,29 and are thus more likely to benefit from plaque modification prior to stent implantation30.
In concert with the progressive ageing of the population, the frequency of severe coronary calcification in patients undergoing PCI, currently estimated to range between 18 and 26%, is likely to grow31,32,33. This is of clinical significance as the presence of severe calcium is a strong predictor of target lesion failure (TLF), late/very late stent thrombosis, major adverse cardiovascular events (MACE) and all-cause mortality in patients undergoing revascularisation33,34.
CORONARY IVL: RATIONALE, PRINCIPLES AND DEVICE DESCRIPTION
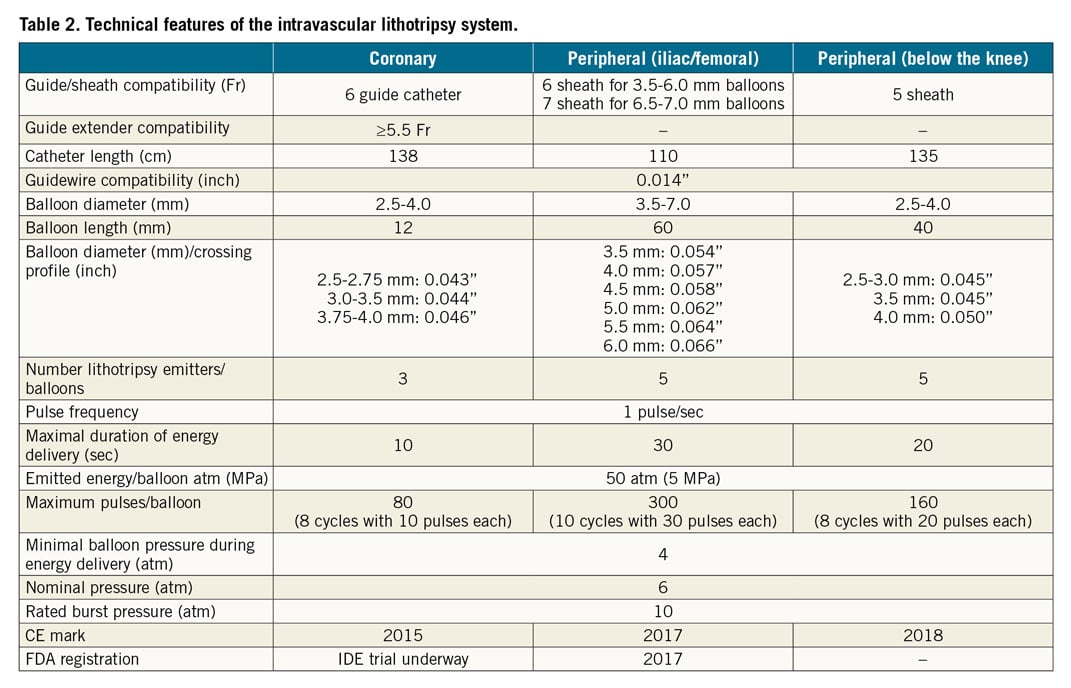
IVL modifies calcific atherosclerotic lesions by inducing calcium fracture before stent deployment with the aim of facilitating drug-eluting stent (DES) expansion and apposition. Coronary IVL follows the same fundamental principles of traditional lithotripsy used for fragmentation of renal calculi, where disruption of calcified tissue using pulsatile mechanical energy is selective to amorphous calcium, minimising soft tissue injury. The second-generation coronary IVL catheter (Shockwave Medical, Santa Clara, CA, USA) is a single-use sterile disposable catheter that contains multiple lithotripsy emitters enclosed in an integrated balloon (Table 2). The emitters create sonic pressure waves in the shape of a sphere, creating a field effect on vascular calcium circumferentially. These sonic pressure waves selectively disrupt and fracture calcium in situ, altering vessel compliance, while minimising injury and maintaining the integrity of the fibro-elastic components of the vessel wall. Compared to contemporary non-compliant balloon crossing profiles (0.033-0.035”), current coronary IVL catheters have a modestly higher profile (0.043-0.046”), similar to contemporary cutting balloons (0.043”). IVL balloon catheters are available in 2.5 to 4.0 mm diameters (in 0.5 mm increments) and 12 mm in length. The IVL catheter is introduced into the target vessel and positioned across the maximally calcified segment of the lesion, using the marker bands as guides, to ensure that the circumferential therapeutic field effect created by the emitters is focused. The IVL catheter is connected via a connector cable to the generator that is pre-programmed to deliver 10 pulses in sequence at a frequency of 1 pulse/second for a maximum of 80 pulses per catheter. The IVL catheter should be sized 1:1 to the reference vessel diameter, inflated to sub-nominal pressures, typically 4 atmospheres (atm), to allow contact with the vessel wall but minimise static barotrauma. If the IVL catheter is unable to pass into the lesion, adjunctive therapies such as predilatation, buddy wire, guide catheter extensions or atherectomy may be performed. Following delivery of a pulse cycle, a brief further dilatation to nominal pressures (6 atm) or higher may be performed if full expansion has not been achieved. IVL treatment is repeated along the target lesion with interval deflation after 10 pulses to allow distal perfusion and overlap of long calcified segments as needed. If the maximum number of pulses per catheter is delivered but lesion preparation is incomplete, further IVL catheters with the same or different diameters may be used (Table 1).
After coronary IVL is performed, intravascular imaging may aid in the detection of the desired calcium fracture within the target lesion (Figure 1, Figure 2). With IVUS, calcium fractures may be subtle, identified as discrete separations across linear luminal echogenicity (Figure 1). Due to its higher resolution, OCT is the technique of choice to identify single or multiple partial or full thickness calcium fractures (Figure 2).
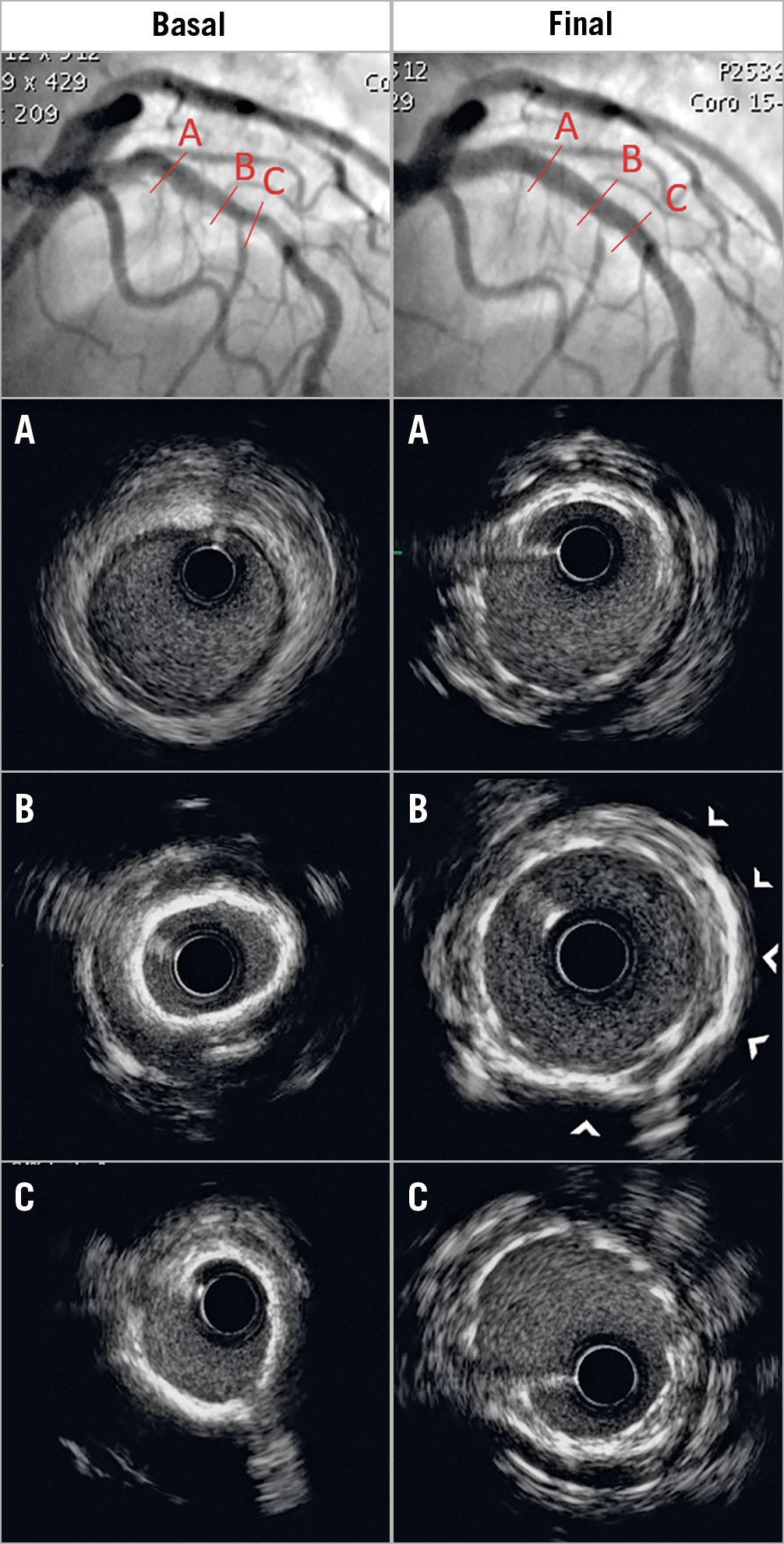
Figure 1. Concentric calcific coronary lesion. Left panels (A, B & C): coronary angiogram with calcific stenosis of the proximal and mid left anterior descending artery (LAD) (upper panel) was confirmed by IVUS (calcium arc 360°; left panel B & C). Coronary IVL was performed using a 3.5×12 mm Shockwave C2 balloon on the distal lesion and a 3.75×12 mm balloon on the proximal lesion. A 3.5×38 mm DES was implanted at 8 atm followed by post-dilation with a 4.0 mm non-compliant balloon at 14 atm. Right panels (A, B & C): angiography shows minimal residual diameter stenosis, confirmed by IVUS showing large acute gain without malapposition. Arrowheads in panel B denote arc segments with reduced shadowing suggesting calcium modification.
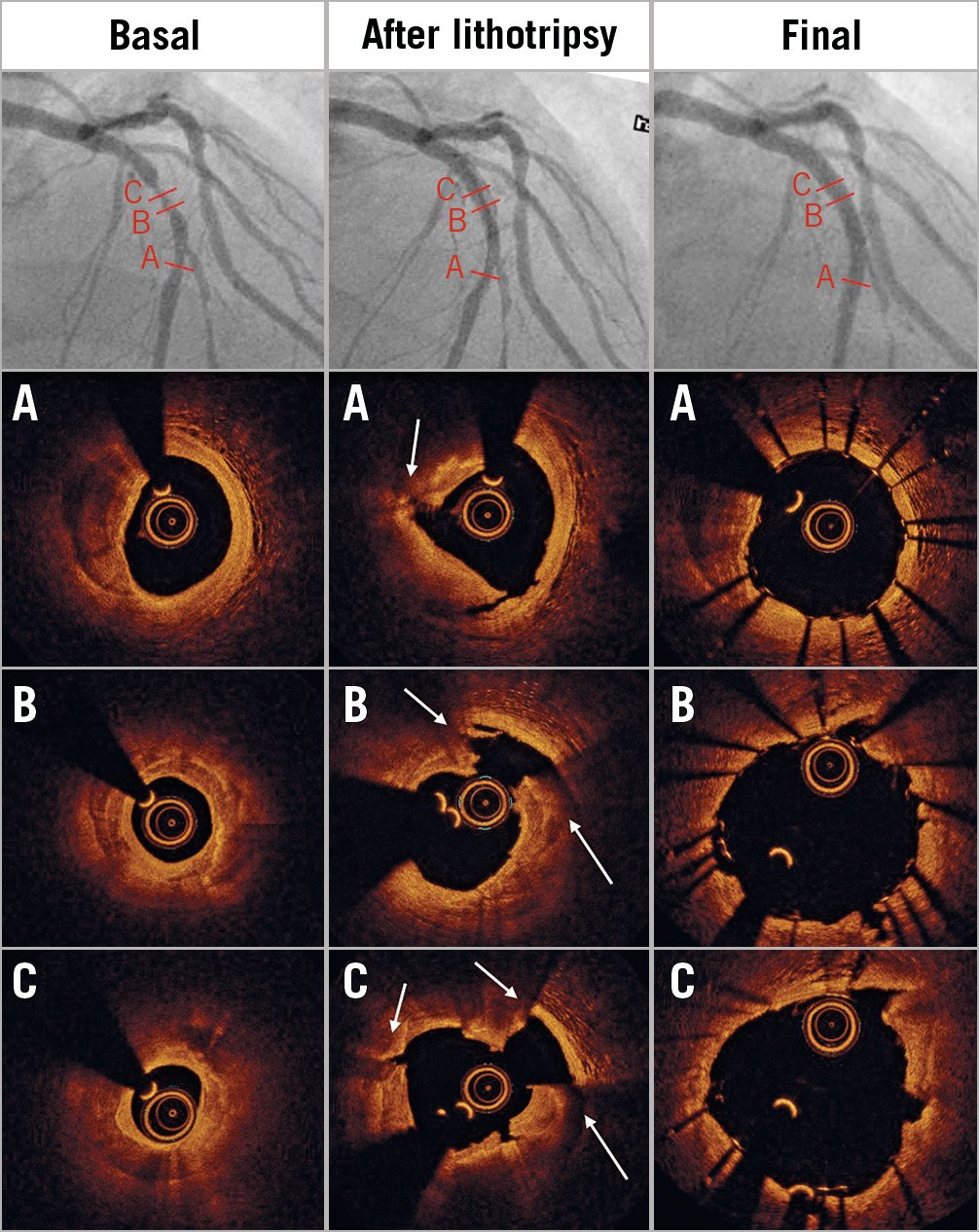
Figure 2. Multiple fractures of a thick calcific coronary plaque. Left panels: coronary angiography identified a long calcific stenosis of the mid left anterior descending artery (LAD). Pre-PCI OCT examination confirmed a severely calcified lesion (MLA 1.1 mm², max calcium thickness 1 mm), with multiple calcium morphologies (A-C). Central panels: 3.5×12 mm and 3.75×12 mm IVL balloons delivered therapy at 4/6 atm. OCT showed multiple fractures (arrows). Right panels: final angiographic and OCT results confirming large luminal gain and minimal malapposition (3.5×18 mm DES deployed at 10 atm).
CLINICAL OUTCOME AND SAFETY OF CORONARY IVL
Disrupt CAD I was the first prospective multicentre, single-arm trial designed to assess the efficacy and safety of coronary IVL in the treatment of calcified coronary lesions (Table 3),34. Sixty patients with de novo moderately or severely calcified coronary stenoses in native vessels were enrolled. Device success was 98.3% and the primary endpoint, residual diameter stenosis <50% after stent implantation without in-hospital MACE (cardiac death/myocardial infarction/target vessel revascularisation [TVR]), was achieved in 95% of patients. IVL was highly effective, achieving acute gains (1.7±0.6 mm) and residual stenosis (13.3±11.6%) similar to those seen in contemporary DES studies comprising largely non-calcified lesions35. The OCT substudy of Disrupt CAD I, in 31 patients treated with serial OCT before and after IVL, showed that the mechanism of IVL was intraplaque calcium fracture. Fracture was identified in 43% of lesions and multiple fractures in >25% of lesions, with a greater number of fractures occurring in the most heavily calcified lesions21.
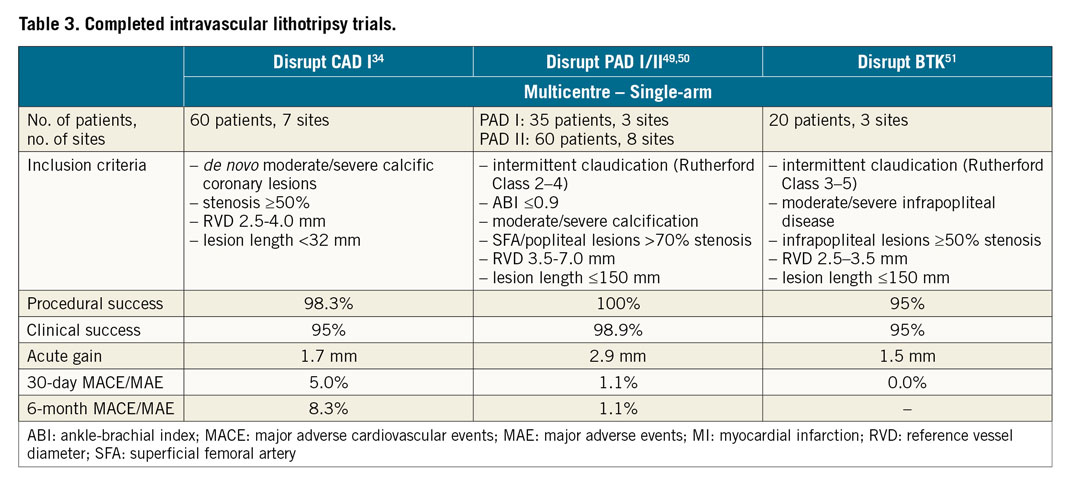
Data derived from clinical trials and from real-world experience of selected centres have consistently shown coronary IVL to be safe. No intraprocedural complications including perforation, abrupt closure, thrombosis or slow flow/no-reflow were described in the trial34. The rate of MACE was 5% and 8% at one and six months, respectively, comprising three non-Q-wave MI and two cardiac deaths deemed unlikely to be related to the index procedure. The absence of vessel perforation, the most fearsome and life-threatening complication of calcific lesions, appears to be a potential major advantage of IVL, but the absence of comparative large trials limits this evidence to anecdotal.
Two IVL studies are currently underway. Disrupt CAD II (NCT03328949), a post-marketing registry, evaluating the safety and performance of coronary IVL in 120 real-world patients is currently enrolling in 15 centres in Europe. Disrupt CAD III (NCT03595176) is a prospective, multicentre, single-arm, global investigational device exemption (IDE) study of 392 patients at 50 centres evaluating IVL for treatment and device-related adverse events (cardiac death/MI/TVF). Similar to Disrupt CAD I, this study will also have an OCT substudy focused on understanding the IVL mechanism of action further.
In addition to these clinical studies, a growing body of anecdotal clinical evidence is emerging. Several case reports discussing the use of IVL in underexpanded stents, where the reason for the underexpansion is calcium, secondary to inadequate vessel preparation, have been reported36,37,38,39.
Calcific peripheral stenoses
Peripheral IVL may be used for the treatment of moderate-severe calcific lesions in different sites with a variety of indications. The rationale, indications and clinical outcome of IVL in peripheral stenosis are discussed in the online supplementary data (Supplementary Appendix 1, Supplementary Figure 1, Supplementary Figure 2).
Conclusion
Both in coronary and peripheral arteries, IVL delivered at low atmospheric pressures can circumferentially fracture calcium, augmenting expansion in severely calcified lesions. IVL is an appealing alternative to more invasive surgical approaches to insert large catheters for TAVI, thoracic endovascular aortic repair (TEVAR) or mechanical circulatory support. Data from large real-world applications and controlled comparisons with the other available treatments are necessary to understand the anatomic characteristics of the calcified lesions with the optimal response. Future iterations of IVL which fine-tune energy delivery to increase efficacy in particularly thick calcium plates while maintaining safety are awaited.
Conflict of interest statement
Z. Ali reports equity in Shockwave. He has also received grants and personal fees from Abbott, Medtronic and Cardiovascular Systems Inc., and personal fees from Boston Scientific and Cardinal Health, outside the submitted work. C. Sorini Dini, B. Tomberli, A. Mattesini, F. Ristalli, S. Valente, M. Stolcova, F. Meucci, G. Baldereschi, F. Fanelli and C. Di Mario work in the Careggi Hospital which has been assigned a research grant to conduct the Disrupt CAD II trial, sponsored by Shockwave. C. Di Mario is the worldwide principal investigator of the Disrupt CAD II trial. F. Fanelli has participated in the DISRUPT PAD trial. The other author has no conflicts of interest to declare.

