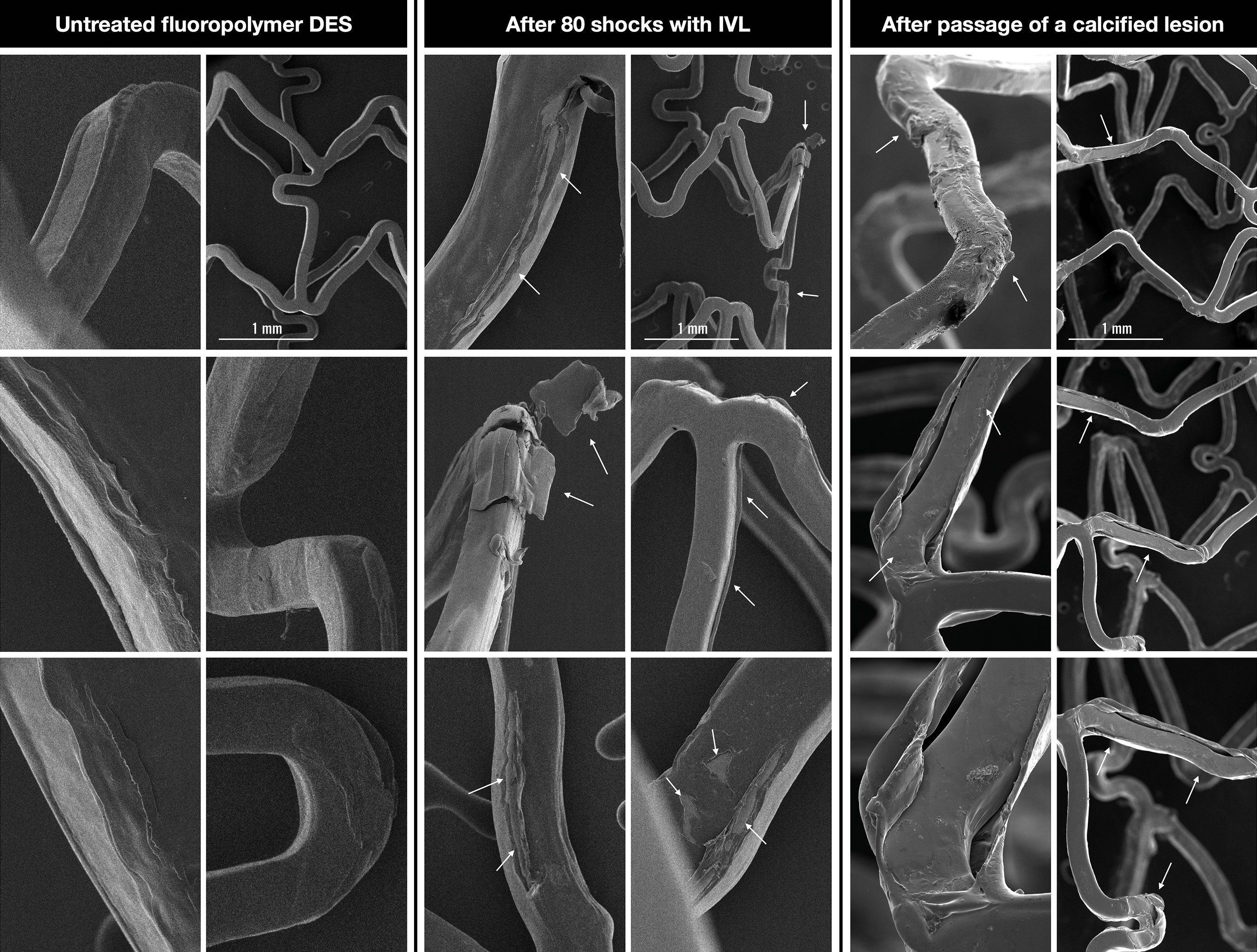
Figure 1. Comparison of the effect of lithotripsy and a resistant calcified lesion on the fluoropolymer of an everolimus-eluting stent. DES: drug-eluting stent; IVL: intravascular lithotripsy
Intravascular lithotripsy (IVL) delivers localised pulsatile sonic pressure waves to circumferentially disrupt vascular calcium with promising results1. Although initially introduced for lesion preparation and plaque modification, it has recently been used for the treatment of underexpanded drug-eluting stents (DES)23. However, no clear evidence exists as to whether the sonic pressure waves affect the polymer and, subsequently, the antiproliferative drug of a freshly implanted DES. This may result in downstream effects, such as distal embolisation of polymer fragments and impaired drug deposition, with increased risk of in-stent restenosis (ISR).
In a study of underexpanded DES secondary to heavily calcified lesions, IVL was used as bailout treatment immediately after stenting in over 40% of the cases and was shown to be fairly ineffective in this situation for increasing lumen and stent dimensions2. While IVL seems to partially overcome the mechanical restraints to enable full expansion of the stent and achieve an acceptable minimum stent area (MSA) concern was raised that it may disrupt the polymer and the antiproliferative agent and, thus, actually promote ISR. We conducted a bench test to address the mechanical effects of IVL on the polymer of a DES.
Eighty IVL shocks were delivered to a 3.5x18 mm everolimus-eluting stent coated with fluoropolymer (XIENCE Skypoint; Abbott), implanted at nominal pressure in a silicone vessel model submerged in water (Moving image 1). The stent was analysed by scanning electron microscopy (SEM). An identical DES, expanded at nominal pressure but not exposed to IVL, was also analysed by SEM for comparison. The rationale for using this particular stent was that the polymer is durable and completely coats the stent, unlike with other designs where a bioresorbable or only abluminal coating is used. Our findings are highlighted in Figure 1. Small tears, microcracks and detachments of polymer flakes can be observed on both the luminal and abluminal side of the DES that had been subjected to IVL (arrows). However, the overall integrity of the fluoropolymer coating remains preserved and, theoretically, no significant reduction of the antiproliferative drug should occur. The control DES showed minor imperfections and uneven surface areas created during the fluoropolymer spray-coating process, but none of the disruptive features seen in the IVL-treated DES.
Microlesions in the fluoropolymer are not solely caused by the effect of IVL: forcing the stent through a tight, calcified stenosis can also lead to similar polymer disruptions. To compare the damage in these 2 situations, we performed a third experiment, post-dilating ex vivo the same type of DES in an extremely calcified popliteal artery specimen. Aiming to simulate a very tight stenosis, the artery was additionally compressed from the outside with a clamp (Moving image 1). The stent, which could only be passed with extreme difficulty owing to much friction, was implanted at nominal pressure and then post-dilated at high pressure with a 4.0 mm non-compliant (NC) balloon. The artery was incised longitudinally; the stent was then extracted and analysed by SEM. Figure 1 shows similar disruptions, even more extensive than in the case of IVL. The extent of the defect depends very much on the type of polymer. Indeed, Wiemer et al found among various failed implantation stent platforms that the one with the durable fluoropolymer was the most stable 4.
In conclusion, IVL does interfere with the fluoropolymer of DES but not enough to significantly reduce the antiproliferative drug and potentially promote ISR. The changes are not unique; they also occur after high-pressure dilatation and especially during interaction with a tight calcified lesion. Severe underexpansion resulting in an unfavourable MSA is the predominant driver of ISR and may justify the bailout use of IVL in this scenario. Overall, the benefit of not leaving an underexpanded stent behind may outrun the impaired local antiproliferative drug effects of the minimally damaged polymer. Analysis of longer-term outcomes assessing target lesion revascularisation and identifying ISR in the international multicentre CRUNCH registry2 may answer this question. However, state-of-the-art lesion preparation, possibly also including IVL, before stent implantation is strongly recommended and may prevent both mechanisms of interaction with the polymer.
As a limitation, we acknowledge that our results apply only to the durable fluoropolymer found on the DES in question and that the in vitro effects of IVL may be different from those in vivo, where the stent is underexpanded.
Whereas the clinical impact of IVL on the polymer coating may be small, the more important aspect is whether the stent’s metallic scaffold absorbs the sonic energy and limits the effect on the calcium underneath. Findings from the SMILE registry support this assumption with a lower IVL efficacy in multilayer stents 3. The importance of calcium modification and lesion preparation cannot be emphasised more, also taking into account that there are other means of calcium modification such as non-compliant super high-pressure, cutting, and scoring balloons, as well as rotational and orbital atherectomy that are all prevented after stent deployment. Intracoronary imaging is advisable and may direct the ideal strategy for calcium modification and prevent stent underexpansion. However, IVL could serve as a bailout option.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. In-bench application of IVL shocks and the simulation of a nearly uncrossable stenosis.

