
Introduction
Intravascular lithotripsy (IVL; Shockwave Medical, Santa Clara, CA, USA) is a new treatment for coronary artery calcification (CAC) that may offer advantages over existing balloon-based therapies and atherectomy devices. Use of IVL in calcific non-left main (LM) disease has been shown to be safe with high acute gain and low residual stenosis1. Similar benefits might be expected in patients with calcific distal LM disease but have yet to be reported.
Methods
This was a retrospective analysis of all patients with obstructive calcific distal LM (or equivalent) disease treated with IVL in three tertiary centres between May 2018 and April 2019. Patients were included if IVL had been performed in ≥1 undilatable segment (distal LM, ostial left anterior descending [LAD] or ostial left circumflex [LCX]) with severe calcification (>270° arc of calcium) confirmed on intravascular imaging. An exclusion criterion was cardiogenic shock. All patients had a clinical indication for revascularisation, received coronary IVL as part of standard care and provided written informed consent prior to intervention.
ENDPOINTS
The primary efficacy endpoint was minimum stent area (MSA) stratified by segment. Secondary efficacy endpoints included minimum stent diameter (MSD), residual area stenosis (%) and relation of MSA to the accepted target value of 5.0 mm2, 6.3 mm2 and 8.2 mm2 for the ostial LCX, ostial LAD and distal LM, respectively2. The co-primary safety endpoints were the incidence of periprocedural complications and major adverse cardiac events (MACE) at 30 days, defined as all-cause death, non-fatal myocardial infarction (MI) or target vessel revascularisation.
IMAGE ANALYSIS
Qualitative and quantitative analysis of intracoronary imaging was performed on a segmental basis. The maximum arc of calcium, reference minimum lumen area (MLA) and reference minimum lumen diameter (MLD) were recorded for each segment prior to IVL. MSA and MSD were measured after stent implantation and optimisation.
DEFINITIONS
Supplementary Appendix 1.
STATISTICAL ANALYSIS
Continuous data are expressed as mean±standard deviation; categorical data are expressed as total number and percentage unless otherwise stated. An unpaired t-test was used to compare MSAs between different groups.
Results
We identified 31 consecutive eligible patients, representing 14% of the total LM percutaneous coronary intervention (PCI) procedures undertaken. Patient characteristics are outlined in Table 1. The distribution of disease and use of IVL are illustrated in Figure 1. In 65% (n=20) of patients, IVL was performed in >1 segment. The final balloon prior to switching to IVL was a semi-compliant, non-compliant and cutting balloon in 16, 14 and 1 patient, respectively. In 2 patients, rotablation also failed to modify the lesion adequately. Sixty-five percent (n=20) of patients had repeat intracoronary imaging performed after IVL and prior to stenting. In all of these cases, multiple calcium fractures were identified (Figure 2).
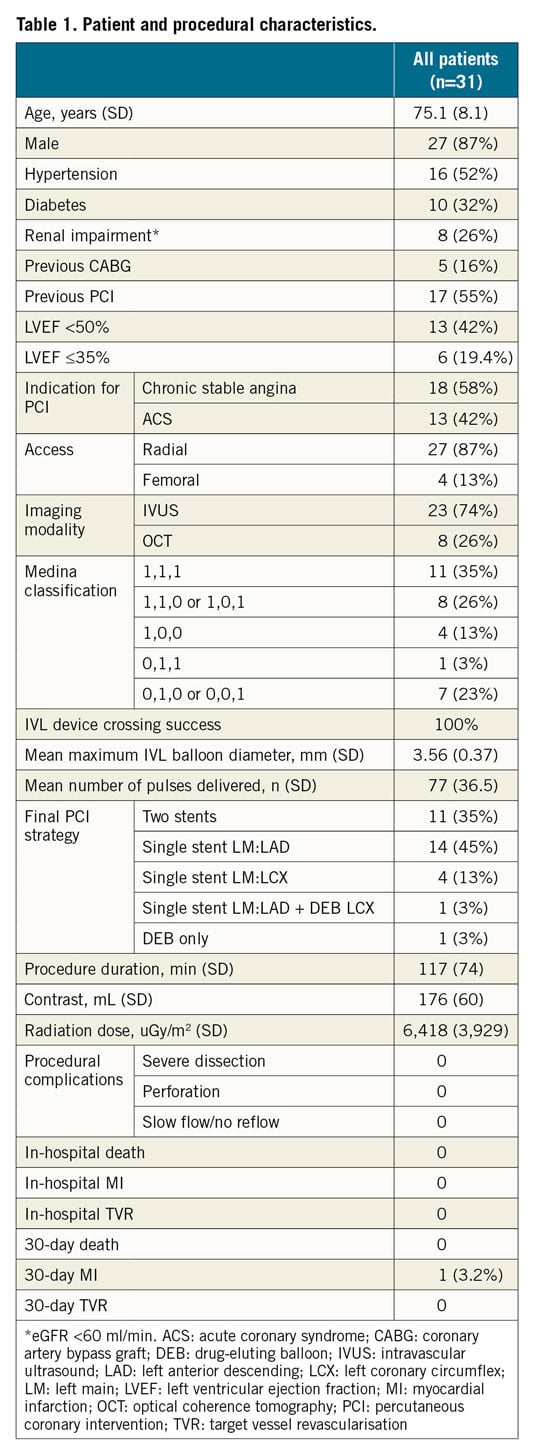
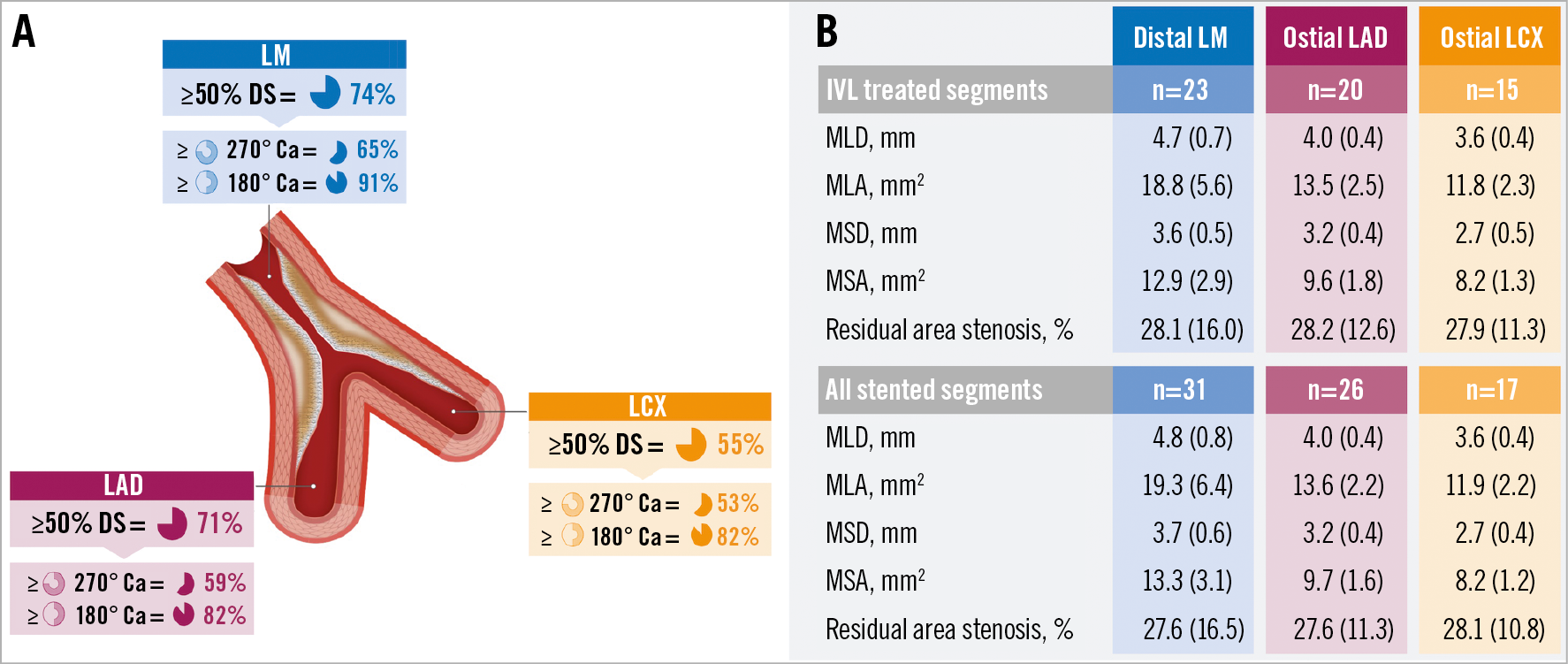
Figure 1. Mechanical outcomes. A) Distribution of obstructive and calcific disease. B) Mechanical outcomes classified according to each segment. Ca: calcium; DS: diameter stenosis; IVL: intravascular lithotripsy; LAD: left anterior descending; LCX: left coronary circumflex; LM: left main stem; MLA: minimum lumen area; MLD: minimum lumen diameter; MSA: minimum stent area; MSD: minimum stent diameter
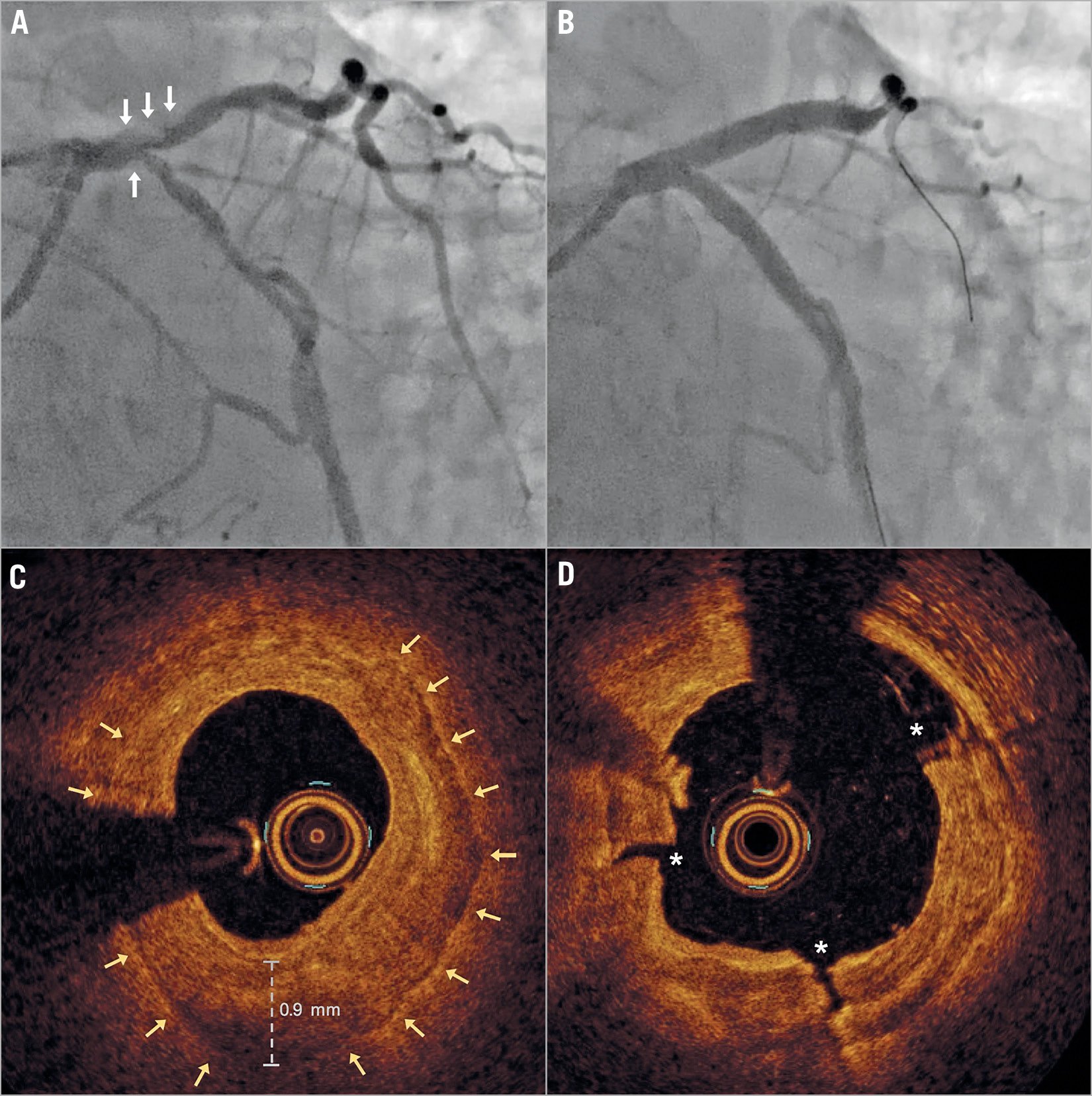
Figure 2. Representative case example. A) Pre- and (B) post-PCI angiographic images, and (C) pre- and (D) post-IVL OCT images of the distal LM. Note the thick plate of near concentric calcification in panel C with multiple fractures (D) following IVL.
All patients underwent successful PCI with stent implantation (97%) and/or drug-eluting balloon (6%). Culotte was the only two-stent strategy used. Proximal optimisation technique (POT) and post-dilatation with non-compliant balloons were performed in all cases. In all two-stent cases, final kissing inflations were performed. Up-front mechanical support with the Impella® device (Abiomed, Danvers, MA, USA) was used in two of the patients with severe left ventricular systolic dysfunction (LVSD) (LVEF ≤35%). One of these patients was intermittently dependent on mechanical support but this was not specifically related to IVL. The second patient had no issues and none of the other patients with severe LVSD had any significant haemodynamic instability.
OUTCOMES
Mechanical outcomes are detailed in Figure 1. Target MSAs were achieved in 96.6% (56/58) and 97.3% (72/74) of all IVL treated and stented segments, respectively. MSAs for segments treated with IVL that were undilatable with either a non-compliant or cutting balloon (LM, 12.0; LAD 9.1; LCX 7.5 mm2) were marginally lower than those undilatable with a semi-compliant balloon (LM 14.1 mm2; LAD 10.0 mm2; LCX 8.8 mm2), but not significantly different (p=ns for all). Coronary IVL was not associated with any procedural complications or in-hospital adverse events (Table 1). The 30-day MACE rate was 3.2% (n=1). The single recorded event was a non-ST-elevation MI from plaque rupture in a non-target vessel (RCA), confirmed on coronary angiography.
Discussion
The immediate mechanical result of PCI has a strong influence on outcomes2,3. However, for many patients, achieving adequate stent deployment remains a major issue owing to the presence of CAC. The DISRUPT CAD I and II trials demonstrated the utility of IVL in patients with calcific non-LM coronary disease1. Our results provide the first evidence that IVL may be equally effective in the treatment of undilatable calcific distal LM disease. High mean MSAs were achieved in each of the distal LM segments, including the LCX ostium, with only two patients failing to meet accepted target values in all treated vessels2. The absence of a comparator group and modest sample size limit any conclusions. Nevertheless, it is noteworthy that, despite the ubiquitous presence of severe undilatable calcific disease, mechanical outcomes in this study compare favourably to other contemporary LM data. In the EXCEL and NOBLE trials the mean distal LM MSAs were 9.8 mm2 and 12.5 mm2, respectively, whereas in the current study it was 12.9 mm2 (Maehara A. et al. Impact of final minimal stent area by IVUS on 3-year outcome after PCI of left main coronary artery disease: the EXCEL trial. Presented at the American College of Cardiology Scientific Sessions, Washington, DC, USA, 18 March 2017)4.
CAC is associated with a higher incidence of periprocedural complications including embolisation and myocardial infarction3. Given the large area of myocardium subtended, minimising this risk is of particular importance in LM PCI. Coronary IVL uses shockwaves to disrupt calcium selectively and is performed at low atmospheric balloon pressure (4-6 atmospheres). This mechanism of action is distinct from other traditional therapies and may have advantages in terms of improving procedural safety1. Consistent with this potential, we observed no periprocedural complications and no target vessel-related events at 30 days. However, we emphasise that our results do not indicate that IVL removes the need for adjunctive supportive measures. These should continue to be used as dictated by clinical status, coronary anatomy and LV function.
Limitations
The main limitations of this study are the modest sample size and lack of a comparator group.
Conclusion
Coronary IVL appears to be a safe and effective treatment for undilatable calcific distal LM disease. Further trials are warranted to establish longer-term outcomes and the benefit of IVL over existing calcium-modification therapies.
|
Impact on daily practice Left main coronary artery disease is increasingly treated percutaneously; however, overcoming severe calcification remains an issue. This study demonstrates that coronary intravascular lithotripsy appears to be a safe and effective treatment for undilatable, severely calcified distal left main disease. |
Acknowledgements
Vascular Perspectives assisted with the graphic design of Figure 1.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

