Abstract
Background: New ischaemic brain lesions on magnetic resonance imaging (MRI) are reported in up to 86% of patients after transcatheter edge-to-edge repair of the mitral valve (TEER-MV). Knowledge of the exact procedural step(s) that carry the highest risk for cerebral embolisation may help to further improve the procedure.
Aims: The aim of this study was to identify the procedural step(s) that are associated with an increased risk of cerebral embolisation during TEER-MV with the MitraClip system. Furthermore, the risk of overt stroke and silent brain ischaemia after TEER-MV was assessed.
Methods: In this prospective, pre-specified observational study, all patients underwent continuous transcranial Doppler examination during TEER-MV to detect microembolic signals (MES). MES were assigned to specific procedural steps: (1) transseptal puncture and placement of the guide, (2) advancing and adjustment of the clip in the left atrium, (3) device interaction with the MV, and (4) removal of the clip delivery system and the guide. Neurological examination using the National Institutes of Health Stroke Scale (NIHSS) and cerebral MRI were performed before and after TEER-MV.
Results: Fifty-four patients were included. The number of MES differed significantly between the procedural steps with the highest numbers observed during device interaction with the MV. Mild neurological deterioration (NIHSS ≤3) occurred in 9/54 patients. New ischaemic lesions were detected in 21/24 patients who underwent MRI. Larger infarct volume was significantly associated with neurological deterioration.
Conclusions: Cerebral embolisation is immanent to TEER-MV and predominantly occurs during device interaction with the MV. Improvements to the procedure may focus on this procedural step.
Introduction
Transcatheter edge-to-edge repair (TEER) of the mitral valve with the MitraClip system (Abbott) is an approved endovascular treatment option for patients with severe mitral regurgitation who carry a high risk for surgery-associated complications12. However, cardiac endovascular treatments may be associated with clinically overt stroke and imaging-based silent brain ischaemia345. Major MitraClip studies reported low rates of clinically overt strokes at 0.2% to 1.2% for in-hospital stroke and 0.7% to 2.6% for stroke within 30 days5. Apart from clinically overt stroke, new ischaemic brain lesions on magnetic resonance imaging (MRI) occurred in up to 86% of patients after TEER of the mitral valve with the MitraClip system67. These new ischaemic brain lesions may be linked to memory loss, cognitive decline, and dementia48. In a prior study testing a cerebral protection system during the MitraClip procedure, debris captured from these filter-based cerebral embolic protection devices was identified in all examined patients9.
TEER of the mitral valve with the MitraClip system entails several procedural steps that could cause cerebral embolisation. However, the procedural step(s) that carry the highest risk for cerebral embolisation remain unclear. Microembolic signals (MES) measured by transcranial Doppler (TCD) are an established online biomarker for thromboembolic complications101112. In this prospective, observational study with pre-specified outcomes, all participating patients underwent continuous TCD examination during TEER of the mitral valve to identify procedural steps with an increased risk for cerebral embolisation. In addition, clinical (neurological and neuropsychological) and cerebral MRI examinations were performed both before and after the procedure.
Methods
Patients and study design
Patients with heart failure and moderate/severe mitral regurgitation treated with TEER of the mitral valve using the MitraClip system were included in this observational study (the STROBE checklist, Supplementary Appendix 1). Recruitment was performed prospectively at the Charité – Universitätsmedizin Berlin, Campus Benjamin Franklin, between June 2017 and September 2019 (ClinicalTrials.gov: NCT03104556). Exclusion criteria were: <18 years of age, inability to consent, pregnancy, or participation in an interventional trial. The study was approved by the local Ethics Committee of the Charité – Universitätsmedizin Berlin, Germany (EA2/005/17). All patients gave written informed consent.
All participating patients underwent continuous TCD examination during TEER of the mitral valve. In addition, clinical and cerebral MRI examinations were performed before and after the procedure.
MitraClip procedure, assessment of mitral regurgitation
In all patients, intracardiac sources of cerebrovascular embolism (especially thrombus) were excluded by transoesophageal echocardiography at the beginning of the procedure. TEER of the mitral valve with the MitraClip system was performed in a standard fashion2. In brief, transseptal puncture was performed after administration of unfractionated heparin (aiming for an activated clotting time [ACT] >250 seconds) under echocardiographic guidance. The distal end of the guide catheter was positioned in the left atrium; the device (MitraClip system, XTR, NT and/or NTR) was steered and rotated with minimum manipulation prior to valve crossing and capture of leaflets. The first clips were passed into the left ventricle with open clip arms, the second and third clips passed into the left ventricle with closed clip arms. Prior to deployment, leaflet insertion and closure were verified with standard manoeuvres. The total procedure time was defined starting with puncture of the femoral vein and ending with removal of the guide catheter and suture-mediated closure of the puncture site. ACT was measured routinely in 30-minute intervals throughout the MitraClip procedure and after every administration of unfractionated heparin.
The severity of mitral regurgitation was graded as mild (I), moderate (II), or severe (III)13.
Transcranial Doppler examination
A continuous TCD examination was performed during the complete MitraClip procedure using a DWL Multi-Dop T2 system (DWL Elektronische Systeme GmbH) with software for MES detection (DWL; Multi-Flow MF software, version 8.27) and 64-point fast Fourier transformation. Detection of MES was based on standard criteria1415. Two pulsed-wave 2-MHz Doppler probes were fixed to the patient’s head with the DiaMon (DWL) system. The Doppler probes were used to insonate the middle cerebral arteries at a depth of 50-58 mm with a sample volume of 10 mm. The detection threshold for MES was adjusted to 9 dB. A high-pass filter was set at 100 Hz. MES were measured automatically at two depths with a distance of 5 mm. To differentiate artefacts, each detected MES was verified off-line after the procedure was completed (an artefact appears in both depths at the same time while an embolus moves through the artery and therefore is measured twice at different times). TCD examination and off-line examination of all recorded MES were performed by two examiners (T.B. Braemswig, M. Kusserow). Continuous TCD examination on both sides simultaneously was not feasible in all patients throughout the complete procedure. In a prior study, no difference had been detected between the number of MES in the right and left middle cerebral arteries during transcatheter aortic valve replacement11. Therefore, in this study only MES on one side (right or left middle cerebral artery) of each patient were analysed depending on better signal quality throughout the procedure. MES during TEER of the mitral valve with the MitraClip system were counted separately during the following procedural steps (to reflect the total burden of microembolisation during each step): (1) transseptal puncture and placement of the guide, (2) advancing and adjustment of the clip in the left atrium, (3) device interaction with the mitral valve (i.e., crossing of the mitral valve, grasping the mitral leaflets and closing the device), and (4) removal of the clip delivery system and the guide.
Clinical data
Baseline characteristics of all patients were collected from the medical records. Patients underwent study-specific neurological and neuropsychological examinations before and after the procedure when general anaesthesia or conscious sedation was completely reversed. Neurological endpoints were assessed on the basis of consensus recommendations4. Neurological status was assessed using the National Institutes of Health Stroke Scale (NIHSS), a 15-item impairment scale assessing level of consciousness, gaze, vision, facial palsy, extremity weakness, limb ataxia, sensory loss, language, and dysarthria16. At the time of follow-up clinical examination, the certified examiners (T.B. Braemswig, M. Kusserow, M. Fritsch, H. Erdur) were blinded to the MRI results. Overt stroke was defined as deterioration in the NIHSS ≥1 point(s) after TEER of the mitral valve. Neuropsychological status was assessed using the Montreal Cognitive Assessment (MoCA; cut-off for mild cognitive impairment [MCI] <26)17.
Cerebral MRI
We conducted two cerebral MRI examinations: one before and one after TEER of the mitral valve. The examinations were performed on 3-Tesla MRI scanners (Tim Trio, Skyra and Prisma Fit, all Siemens). The protocol included the following three sequences: diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR), and T2*-weighted imaging. MRI images were reviewed by experienced raters (T.B. Braenswig, K. Villringer, I. Galinovic, J.B. Fiebach). The supervising raters (K. Villringer, I. Galinovic, J.B. Fiebach) were blinded to clinical information. Occurrence, number, volume (in ml) and location of acute DWI lesions (with low apparent diffusion coefficient values) that newly occurred after TEER of the mitral valve were assessed. For volumetric quantification, new DWI lesions were delineated manually in each slice and the volume was calculated using the software MRIcron (NITRC, version 1.0.20190902). Three different vascular territories were differentiated: territory of the left internal carotid artery, territory of the right internal carotid artery, and the vertebrobasilar territory. Vascular territories were defined according to established patterns18. Intracerebral haemorrhage (>10 mm in diameter) was detected on T2*-weighted imaging. Chronic ischaemic lesions and severity of white matter disease (using the Wahlund visual scale19) were assessed on FLAIR.
Statistical analysis
The Wilcoxon test was used for continuous variables. The chi-squared test was used for categorical variables. Univariate linear regression analysis was used to identify procedure-related factors and structural anomalies of the mitral valve associated with an increased number of MES. We used a two-sided significance level of 0.05. Group comparisons (comparing median number of MES during the different procedural steps) were adjusted according to Holm20. All analyses were performed using R software (R Foundation for Statistical Computing, version 4.0.2).
Results
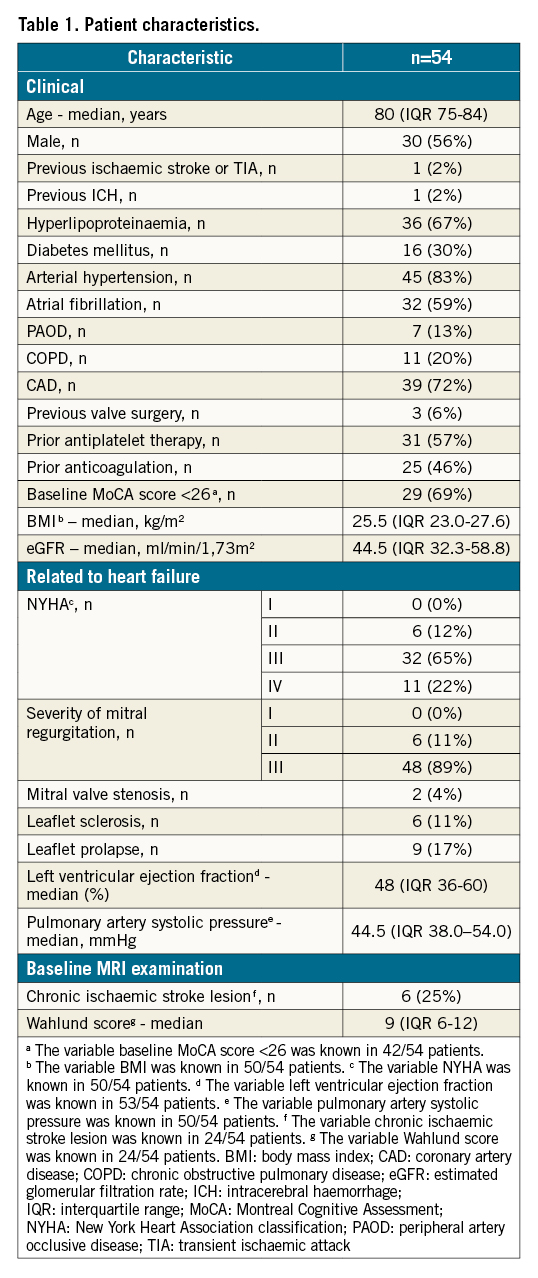
Patient characteristics
Between June 2017 and September 2019, 61 patients with heart failure and moderate to severe mitral regurgitation treated with TEER of the mitral valve using the MitraClip system were included in our study (56% male; median age: 80 years [interquartile range {IQR} 75-84 years]; severity of mitral regurgitation: grade II: 11%, grade III: 89%). Seven patients had to be excluded from the final analysis due to an insufficient acoustic temporal bone window (which did not allow for a continuous detection of MES). Thus, 54 patients were available for the final analysis (Figure 1). The baseline characteristics of these patients are summarised in Table 1. Patients with and without sufficient acoustic temporal bone windows differed significantly regarding previous ischaemic stroke or transient ischaemic attack (2% vs 43%; p=0.001).
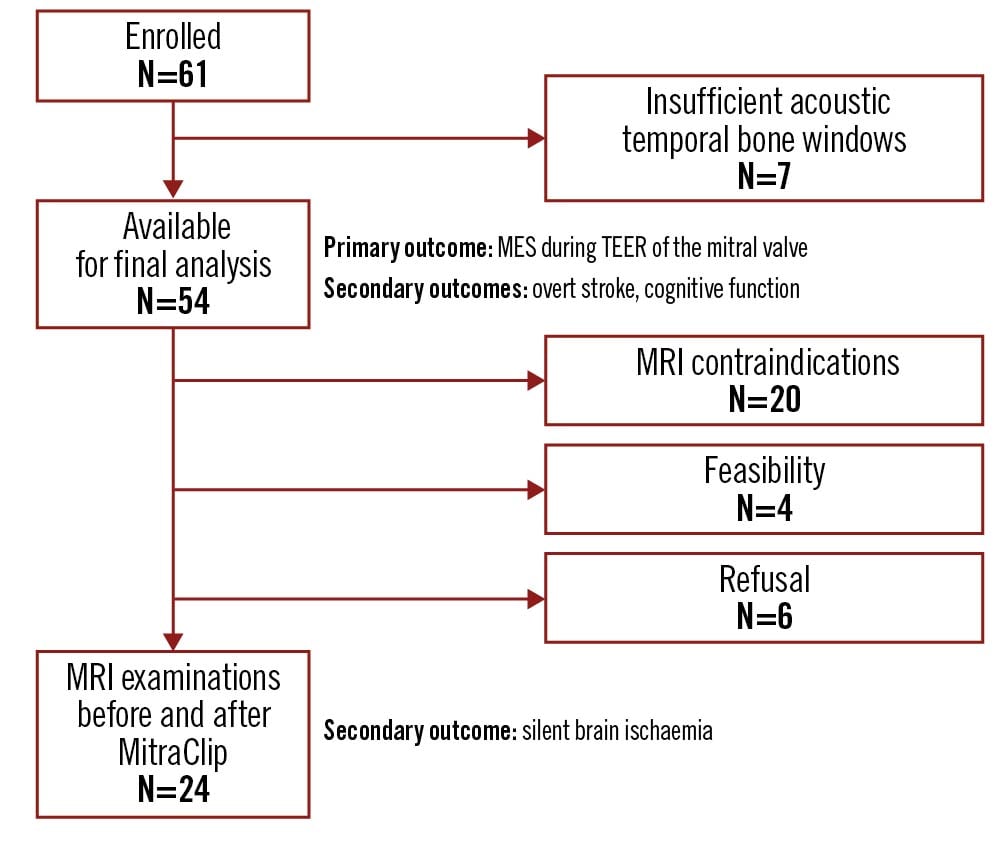
Figure 1. Flow chart of the study population. MES: microembolic signals; MRI: magnetic resonance imaging; TEER: transcatheter edge-to-edge repair
Procedural results
Implantation of the MitraClip system was technically successful in all patients. A reduction of mitral regurgitation was achieved in all patients (severity of mitral regurgitation after the procedure: grade 0: 23%, grade I: 62%, grade II: 15%; the variable severity of mitral regurgitation was known in 53/54 patients). TEER of the mitral valve was performed under conscious sedation in 12 patients, and under general anaesthesia in 42 patients. In 19 patients one clip was implanted, and in 35 patients two or three clips were implanted. The median procedure time was 84 minutes (IQR 71-108 minutes). The median heparin dose administered during the procedure was 11,000 IU (IQR 9,000-13,375 IU). The median ACT was 285 seconds (IQR 262-329 seconds) during the procedure. A cardiac tamponade did not occur in any patient.
Transcranial Doppler examination
MES were observed in all patients during TEER of the mitral valve with the MitraClip system (median number of MES during the complete procedure: 152 [IQR 94-280]). Number of MES differed significantly between the different procedural steps with the highest number of MES observed during device interaction with the mitral valve (Central illustration) (in patients with more than one implanted clip, only MES during implantation of the first clip are presented below):
- (1) transseptal puncture and placement of the guide: 12 MES (median; IQR 5-30)
- (2) advancing and adjustment of the clip in the left atrium: 15 MES (median; IQR 7-26)
- (3) device interaction with the mitral valve (i.e., crossing of the mitral valve, grasping the mitral leaflets and closing the device): 66 MES (median; IQR 32-136)
- (4) removal of the clip delivery system and the guide: 1 MES (median; IQR 0-3).
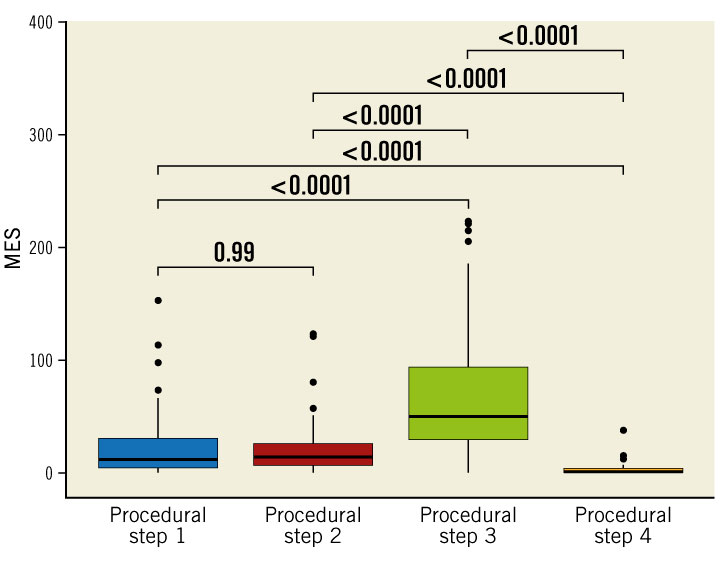
Central Illustration. Number of microembolic signals during the different procedural steps. Procedural steps: (1) transseptal puncture and placement of the guide, (2) advancing and adjustment of the clip in the left atrium, (3) device interaction with the mitral valve, (4) removal of the clip delivery system and the guide. MES: microembolic signals
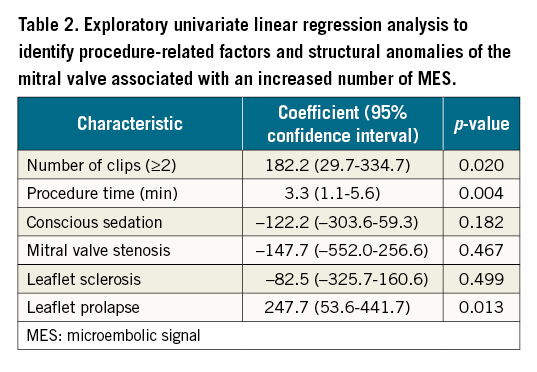
An exploratory univariate linear regression analysis showed that leaflet prolapse, longer procedure time and implantation of ≥2 MitraClips were associated with an increased number of MES (Table 2).
Clinical outcomes
Patients were examined clinically (NIHSS, MoCA) one day before (median; IQR 1-1) and three days after (median; IQR 3-4) TEER of the mitral valve.
A neurological deterioration on the NIHSS ≥1 point(s) equivalent to clinically non-disabling stroke was observed in 9 of the 54 patients following the procedure. Neurological deterioration was equivalent to one point (on the NIHSS) in five patients, two points in three patients and three points in one patient. In other words, neurological deterioration was mild in all patients. New deficits consisted of a mild hemiparesis (arm and/or leg drifted to an intermediate position prior to the end of the full 10 [arm]/5 [leg] seconds) in six patients, a mild-to-moderate sensory loss in one patient, a partial gaze palsy in one patient, an incorrectly answered question in one patient, a mild-to-moderate dysarthria in one patient and an ataxia presented in one limb in two patients. Five out of nine patients with an overt stroke underwent MRI examinations before and after TEER of the mitral valve. Examples of acute, new DWI lesions on MRI that correlated with the described clinical deterioration are shown in Figure 2.
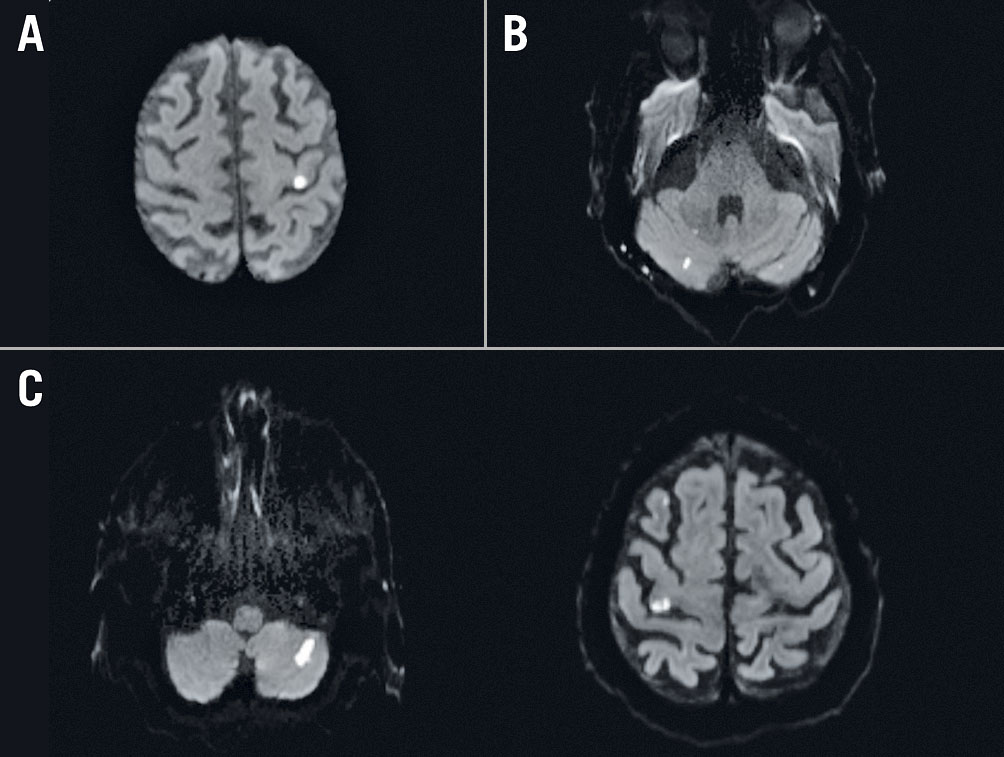
Figure 2. Patients with new non-disabling stroke undergoing cerebral MRI examination after the MitraClip procedure. A. Patient #1: mild right-sided hemiparesis and corresponding acute diffusion-weighted imaging (DWI) lesion (hyperintense) located in the left gyrus precentralis. B. Patient #2: ataxia and corresponding acute DWI lesions located in the cerebellum. C. Patient #3: ataxia and mild left-sided hemiparesis and corresponding acute DWI lesions located in the cerebellum and in the right gyrus precentralis.
In an exploratory univariate analysis, patients with and without overt stroke after TEER of the mitral valve differed significantly (p=0.030) regarding volume of new DWI lesions on MRI (Table 3).
 There was no statistically significant decline in cognitive function after TEER of the mitral valve: median MoCA score before TEER of the mitral valve: 24.00 (IQR 19.00-26.00), median MoCA score after TEER of the mitral valve: 23.00 (IQR 19.75-25.25) (p=0.704 [paired Wilcoxon test]; data were available in 40/54 patients).
There was no statistically significant decline in cognitive function after TEER of the mitral valve: median MoCA score before TEER of the mitral valve: 24.00 (IQR 19.00-26.00), median MoCA score after TEER of the mitral valve: 23.00 (IQR 19.75-25.25) (p=0.704 [paired Wilcoxon test]; data were available in 40/54 patients).
Cerebral MRI
Twenty-four patients received MRI examinations before and after TEER of the mitral valve. Reasons for not undergoing MRI examinations were: MRI contraindication (e.g., pacemaker; 20/30 patients), feasibility (e.g., claustrophobia, obesity; 4/30 patients) and refusal (6/30 patients). Patients were examined on MRI one day before (median; IQR 1-2) and three days after (median; IQR 3-4) TEER of the mitral valve.
New DWI lesions occurred in 21/24 patients after TEER of the mitral valve. The median number of new DWI lesions was seven (IQR 3-13). In all patients with new DWI lesions, lesions were located in more than one vascular territory involving the cerebral cortex. The median volume of new DWI lesions was 0.16 ml (IQR 0.07-0.48 ml).
No intracerebral haemorrhage was detected on follow-up MRI examination.
Discussion
Cerebral embolisation poses an immanent risk in TEER of the mitral valve. In this study, cerebral embolisation was detected in all patients undergoing TEER of the mitral valve with the MitraClip system although the procedure was performed state-of-the-art, resulting in a reduction of mitral regurgitation in all patients. For the first time, we identified the process of device interaction with the mitral valve as the specific procedural step that is associated with the highest number of (micro-)embolisations. The clinical relevance of this finding is demonstrated by the detection of non-disabling stroke in 9 of the 54 patients. The finding itself is supported by imaging-based ischaemic brain lesions in 21 in the subgroup of 24 patients able to undergo MRI.
TCD examination is an established diagnostic tool to monitor embolisation (MES) during cardiac procedures. Thereby, TCD can assign MES to specific steps of the procedure1121. In this study, MES were observed in all examined patients, occurred predominantly during device interaction with the mitral valve and were associated with longer procedure time and implantation of ≥2 MitraClips. This strong association between embolisation and device interaction with the mitral valve is also supported by a previous histopathological analysis that captured debris using a cerebral protection system during MitraClip procedures: besides acute thrombus and foreign material, the debris was most often composed of valve/atrial wall tissue9. Furthermore, device interaction with the aortic heart valve has already been shown as a risk factor for cerebral embolisation in previous studies: Omran et al reported that new ischaemic brain lesions on MRIs occurred after valve passage in patients who underwent retrograde catheterisation of a stenotic aortic valve for diagnostic haemodynamic evaluation22. Kahlert et al reported an increased risk for cerebral embolisation while stent prostheses were positioned and implanted during transcatheter aortic valve replacement11.
In this study, non-disabling stroke occurred in 9 of 54 patients after the MitraClip procedure. In patients who also underwent cerebral MRI examinations, new DWI lesions corresponded neuroanatomically to new neurological symptoms (Figure 2). The incidence of in-hospital stroke in our study was higher than previously reported in several MitraClip registries5. The variation is very likely explained by higher sensitivity due to thorough examination by neurologists who also detected mild deteriorations. This finding is supported by growing evidence that the incidence of periprocedural overt strokes in patients undergoing cardiovascular interventions is under-reported. In general, systematic evaluation by neurologists has shown significantly higher rates of new overt strokes after cardiovascular interventions with mostly mild new deficits4232425. Of note, all patients with a clinical deterioration had solely mild new neurological symptoms (NIHSS ≤3) in our study. Interestingly, in another, much smaller study in which patients were also examined by neurologists, mild new neurological symptoms were detected in 2 of 13 patients after TEER of the mitral valve with the MitraClip system7 – a rate similar to our findings.
In an exploratory univariate analysis comparing patients with and without new overt stroke, volume of new DWI lesions was the only variable that differed significantly between the two groups (patients with overt strokes had a significantly larger volume of new DWI lesions). This is in line with a previous study investigating stroke after aortic valve surgery that also showed an association between overt stroke and larger infarct volume on MRI23. Although not statistically significant, none of the twelve patients who received conscious sedation had an overt stroke after the procedure. Further studies are needed to compare general anaesthesia and conscious sedation during the MitraClip procedure in more detail. Interestingly, in a prior transcatheter aortic valve replacement study, general anaesthesia (as opposed to conscious sedation) was also associated with an increased risk of mortality and stroke526.
Global cognitive screening using the MoCA score did not reveal a statistically significant decline in cognitive function after TEER of the mitral valve. However, a more comprehensive neurocognitive assessment might be necessary to detect subtle deficits5.
In the subgroup of patients undergoing cerebral MRI examinations both before and after TEER of the mitral valve, new DWI lesions occurred in 21 of 24 patients. All patients with new DWI lesions had a radiographic pattern suggesting an embolic origin (≥2 new lesions, lesions located in more than one vascular territory, lesions involving the cerebral cortex)27. The median total volume of new DWI lesions was very small (0.16 ml). Incidence and median total volume of new DWI lesions were both similar to results found by Blazek et al6.
Limitations
Of note, implantation of the MitraClip system was technically successful in all patients. Anticoagulation was adequate, as reflected by a median ACT of 285 seconds during the procedure. The total procedure time was well in range with reports in the available literature9. Furthermore, by performing clinical and cerebral MRI examinations both before and after TEER of the mitral valve, identification of new overt stroke as well as new silent brain ischaemia was possible. Nevertheless, limitations of our study must be considered. First, results of a single-centre study of 54 patients cannot easily be generalised. Second, although no significant associations between overt stroke and procedure-related factors as well as structural anomalies of the mitral valve (e.g., leaflet sclerosis) were observed in the present study, this cannot be excluded and would require further investigation in a larger cohort. Third, characterisation of emboli (especially unequivocally distinguishing solid and gaseous emboli) using conventional TCD equipment is challenging. This methodology-inherent limitation of TCD was described previously2728. Here, however, most MES occurred during device interaction with the mitral valve, a procedural step with a high probability of solid emboli27 supporting the clinical relevance and meaning of our measurements. Fourth, neurological assessment was performed only at one time point after TEER of the mitral valve. Thus, information on further improvement of the mild neurological deficits remains unclear.
Conclusions
In conclusion, our data show that cerebral embolisation is immanent to TEER of the mitral valve with the MitraClip system in its present form. Device interaction with the mitral valve is a key target to reduce embolisation.
Impact on daily practice
In previous studies, new ischaemic brain lesions on magnetic resonance imaging (MRI) occurred commonly after transcatheter edge-to-edge repair (TEER) of the mitral valve. For the first time, we showed that device interaction with the mitral valve is the most vulnerable procedural step during TEER of the mitral valve. In addition, non-disabling stroke with mild new neurological symptoms after TEER of the mitral valve occurred more often in our study than previously reported in several MitraClip registries. Although further investigations in larger cohorts are required, future improvements of the MitraClip procedure may focus on the procedural step of device interaction with the mitral valve.
Funding
This work was supported by the German Heart Foundation/German Foundation of Heart Research. T. B. Braemswig is a participant in the BIH-Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. M. Endres received funding from DFG under Germany´s Excellence Strategy – EXC-2049 – 390688087, BMBF, DZNE, DZHK, EU, Corona Foundation, and Fondation Leducq.
Conflict of interest statement
H.J. Audebert reports receiving personal fees from Bayer Vital, Boehringer Ingelheim, Bristol Myers Squibb, Novo Nordisk, Pfizer, Daiichi Sankyo and Sanofi. M. Endres reports grants from Bayer and fees paid to the Charité from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis and Pfizer. U. Landmesser reports research grants to the institution from Amgen, Bayer, Novartis. Moderate lecture or advisory fee from Abbott, Amgen, Sanofi, Bayer, Pfizer, Daiichi and Boehringer. J.B. Fiebach reports personal fees from Abbvie, AC Immune, Artemida, Bioclinica, Biogen, BMS, Brainomix, Cerevast, Daiichi-Sankyo, Eisai, F. Hoffmann-La Roche AG, Eli Lilly, Guerbet, Ionis Pharmaceuticals, IQVIA, Janssen, Julius Clinical, jung diagnostics, Lysogene, Merck, Nicolab, Premier Research, and Tau Rx. C.H. Nolte reports lecture fees and/or consultancies from Boehringer Ingelheim, BMS, Bayer, Daiichi Sankyo, Sanofi, Pfizer, Alexion, Abbott and Gore & Ass. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

