Abstract
Aims: This study aimed to assess the incidence and impact of cerebral embolic events after the MitraClip procedure.
Methods and results: Twenty-seven high-risk patients (logistic EuroSCORE I 25±15%) underwent the MitraClip procedure and cerebral diffusion-weighted magnetic resonance imaging (MRI) in median two days before and three days after the procedure. On the same day, neurocognitive function was assessed using the Montreal Cognitive Assessment (MoCA) questionnaire and thorough clinical examination. Comparison of pre- and post-interventional MRI showed that 23 of 27 patients (85.7%) had newly acquired microembolic lesions with in median three (interquartile range 1-9) new lesions per patient. Of these, three patients (11.1%) had lesions with diameter >5 mm. Patients with >3 new cerebral embolic lesions (n=13, 48%) had a lower post-interventional MoCA score in comparison to patients with ≤3 embolic lesions (23.6±3.6 vs. 20.3±4.5; p=0.046) in univariate analysis. Multivariate stepwise regression analysis identified device time as an independent predictor of the number of post-procedural new lesions (p=0.003) and, for reduced post-interventional MoCA score, a low MoCA score at baseline (p<0.001).
Conclusions: The MitraClip procedure results in new ischaemic cerebral lesions in the vast majority of patients. Preliminary data suggest that these lesions are clinically without significant impact on global cognitive function. ClinicalTrials.gov: NCT01288976
Abbreviations
ACT: activated clotting time
ADC: apparent diffusion coefficient
DWI: diffusion-weighted imaging
FLAIR: fluid attenuated inversion recovery sequence
FoV: field of view
IQR: interquartile range
MAP: mean arterial pressure
MoCA: Montreal Cognitive Assessment
MRI: magnetic resonance imaging
T2-TSE: T2-weighted turbo spin echo sequence
TE: echo time
TI: inversion time
TR: repetition time
Introduction
In selected patients with severe mitral valve regurgitation and high surgical risk, endovascular treatment using the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) has increased as an alternative treatment option1-3.
Several studies have shown the effectiveness of the procedure1,3-7. Dislodgement of micro-debris, air, and/or thrombi with subsequent embolic cerebral events is a potential drawback.
Previously, the incidence of clinically apparent periprocedural strokes after endovascular mitral valve repair using the MitraClip has been reported at a range of 0-1%1,3-7. The risk of clinically silent cerebral microemboli is unknown.
Often undiagnosed, clinically silent cerebral embolism can lead to impairment of neurocognitive function and dementia aggravation8-11.
The aim of this prospective study was to assess the incidence and impact of both clinically apparent and silent cerebral ischaemia using serial diffusion-weighted magnetic resonance imaging before and after MitraClip procedure.
Methods
PATIENTS
Between March, 2011, and July, 2012, a total of 54 consecutive high-risk patients with severe symptomatic mitral insufficiency underwent transcatheter mitral valve reconstruction using the MitraClip system. Patients with MRI contraindications were excluded, leading to 30 patients being enrolled in this prospective study (Figure 1). Of these, 27 patients completed periprocedural neurological testing and MRI examination and comprised the final study cohort. The pre-procedural diagnostic work-up included a clinical, neurological, laboratory, echocardiographic and electrocardiographic examination as well as a colour-encoded ultrasound of the carotids.
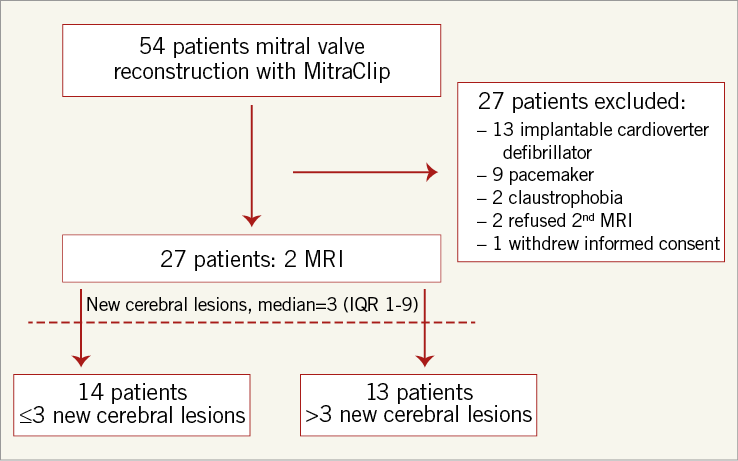
Figure 1. Trial profile. IQR: interquartile range, MRI: magnetic resonance imaging
The study was approved by the local ethics committee and all patients provided written informed consent.
MITRAL VALVE ASSESSMENT
Preoperatively and postoperatively, transthoracic and transoesophageal echocardiography (Vivid 7; GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK) was performed by an experienced cardiologist. The origin and degree of mitral regurgitation was graded according to European Association of Echocardiography recommendations12. Using a scoring system adjusted from the Wilkins score13 the extent of mitral valve calcification was graded into four categories: grade I represents single spot calcification of the leaflets, grade II calcification of the leaflet margins, grade III calcification until the middle of the leaflet, and grade IV calcification of majority of the leaflets. Additionally, qualitative fluoroscopic grading of mitral annular calcifications was performed: grade 0: no visible calcifications; grade 1: isolated spot calcification; grade 2: calcifications extending <50% of the annular circumference; grade 3: calcifications extending ≥50% of the annular circumference; grade 4: circumferential calcifications of the mitral annulus.
MITRACLIP PROCEDURE
The procedure has been described in detail previously2. All procedures were performed under general anaesthesia in a dedicated hybrid operating room. Patients with oral anticoagulation (target international normalised ratio 2.0-3.0) were not required to stop their medication and no bridging was performed. After transseptal puncture, intravenous heparin was administered to achieve an activated clotting time of >250 sec for the entire duration of the procedure. ACT measurement was repeated every 30 minutes. The clip was positioned using 3D transoesophageal echocardiographic (iE33; Philips Healthcare, Best, The Netherlands) and fluoroscopic guidance14. Multiple clip implantations were performed in case of insufficient mitral regurgitation reduction. Procedural success was defined as a reduction of mitral regurgitation to grade ≤2.
An invasively monitored mean arterial blood pressure below 55 mmHg ≥5 minutes was considered a hypotensive event. Operation time was measured in minutes starting with puncture of the femoral vein and ending with the application of the groin pressure bandage. Device time was defined as the moment of inserting the steerable catheter into the guide catheter until delivery of the final MitraClip.
After the procedure, patients were treated with acetylsalicylic acid (100 mg/day) for six months and clopidogrel (75 mg/day) for 30 days or, in case of atrial fibrillation, acetylsalicylic acid was substituted with phenprocoumon (target international normalised ratio 2.0-3.0).
BRAIN MRI
MRI was performed within two days before and two to six days after the intervention, depending on patients’ clinical status. Patients were examined with a 1.5T scanner (Intera; Philips Healthcare) or a 3T scanner (MAGNETOM Verio; Siemens AG, Healthcare Sector, Erlangen, Germany) with a dedicated head coil. In every patient the same scanner was used for pre- and post-interventional imaging. On both scanners a fluid attenuated inversion recovery sequence in axial and sagittal orientation, a T2-weighted turbo spin echo sequence and a diffusion-weighted echo-planar imaging sequence were acquired, each with 25 to 30 slices covering the whole brain. In the 1.5T system the following parameters were used: 1) FLAIR with a repetition time of 10,000 ms, echo time of 140 ms, inversion time of 2,800 ms, flip angle of 90°, field of view of 230×230 mm with matrix of 256×256 pixels and a slice thickness of 5 mm, with an interslice gap of 1 mm in sagittal orientation and no gap in the axial orientation. 2) T2-TSE with TR 6,000 ms, TE 100 ms, 5 mm, no gap, flip angle 90°, FoV: 230×230 mm, matrix: 256×256 pixels. 3) DWI with diffusion gradient b values of 0 and 1,000 s/mm², TR 6,073 ms, TE 88 ms, slice thickness 5 mm, no gap, FoV of 230×230 mm, matrix of 192×192 pixels.
On the 3T system, the following parameters were used: 1) FLAIR transversal and sagittal with TR of 8,000 ms, TE 94 respectively 89 ms, TI 2,173 ms, 5 mm slice thickness, 1 mm gap, FoV 230×230 mm, flip angle 150°. 2) T2-TSE with TR 5,000 ms, TE 91 ms, 5 mm slice thickness, no gap, FoV 230×230 mm. 3) DWI with TR 6,600 ms, TE 100 ms, 5 mm, 1 mm gap, FoV 230×230 mm, flip angle 90° and diffusion gradient b values of 0 and 1,000 s/mm².
Apparent diffusion coefficient maps were calculated automatically for 1.5 and 3T images at the scanner.
Hyperintense lesions in b0 images were correlated with ADC maps to identify newly acquired lesions and to rule out T2 shine-through effects. Image analysis was performed by experienced readers, blinded for clinical data.
Any focal diffusion abnormalities were analysed regarding their number, vascular distribution, and volumetric extension in mm³. The lesion with the highest volumetric extent was defined as the single biggest lesion per patient.
EVALUATION OF NEUROCOGNITIVE FUNCTION
The global cognitive function was assessed with the Montreal Cognitive Assessment (MoCA) questionnaire15 and neurologic testing was determined by thorough clinical examination at admission, and after the procedure daily for three days. The MoCA test was completed on the day of the cerebral MRI studies by a single person blinded to the MRI results.
The MoCA test is a one-page, 30-point test specifically designed for evaluation of mild cognitive impairment. A score of ≥26 is considered as normal. Lower scores indicate more severe neurological impairment15.
STATISTICAL ANALYSIS
Normally distributed data are expressed as mean±SD, not normally distributed data as median and interquartile range (IQR). Proportions are expressed as number of patients and percentages. For further analysis, two groups of patients were created according to the median of new cerebral lesions (group below the median with ≤3 lesions and group above the median with >3 new cerebral bright lesions).
Differences between groups were assessed by Fisher’s exact test for categorical variables and by the Student’s t-test or Mann-Whitney U rank-sum test for continuous data according to their distribution.
Univariable and multivariable linear regression analyses were performed to identify predictors of the number of cerebral lesions and post-interventional MoCA scores. Multivariable regression was performed using only variables with a probability value <0.1 in univariable regression analyses.
The statistical analyses were conducted with the use of commercially available software (MedCalc for Windows, version 12.7.7.0; MedCalc Software, Mariakerke, Belgium). A two-tailed probability value of p<0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
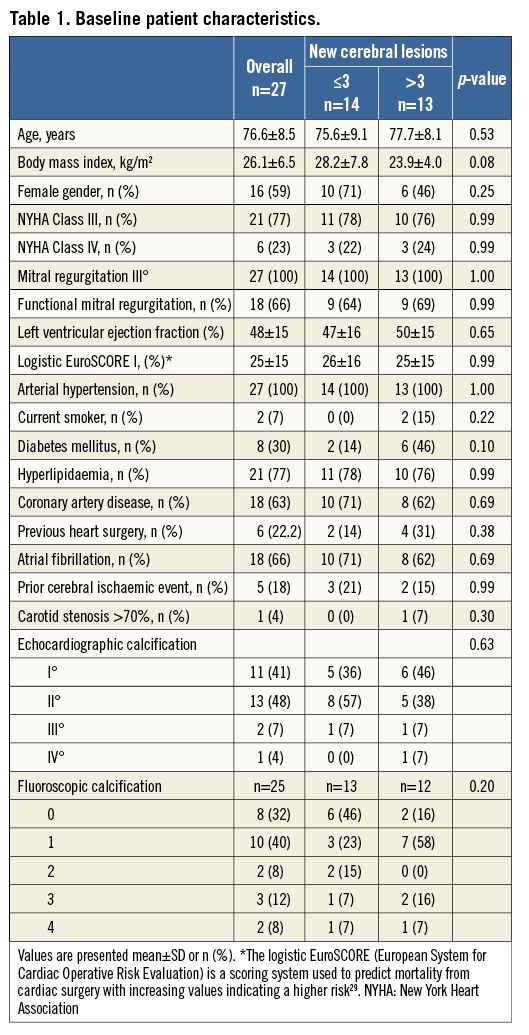
Twenty-seven patients received the complete clinical, neurological and MRI work-up. Our study cohort represents a high-risk cohort considered ineligible for conventional heart surgery by Heart Team assessment reflected by an advanced age (76.6±8.5 years) and a logistic EuroSCORE I of 25±15% with severe mitral regurgitation (Table 1).
Eighteen patients (66.6%) had atrial fibrillation. Prior cerebral ischaemic events were present in five patients (18.5%). Pre-interventional ultrasound revealed asymptomatic stenosis >70% of the left internal carotid artery in one patient.
On preoperative transoesophageal echocardiography, the presence of intracardiac thrombi was ruled out in all patients. There were no significant differences in baseline characteristics in the two groups according to the median of microembolic lesions (Table 1).
MITRACLIP PROCEDURE
The procedural success rate was 96%. In one patient (group >3 embolic lesions) no noteworthy reduction of mitral regurgitation could be observed. Device time was 72±53 minutes and operation time 97±54 minutes. Overall, 32 clips were implanted in 27 patients with a maximum of two clips in five (19%) patients. ACT was 307±62 seconds without any significant differences between treatment groups. In 16 patients interventional hypotensive events (MAP of 56±8 mmHg) could be observed. Of these 16, 14 (87.5%), and an additional three (27%) in the non-hypotensive group, received low-dosed catecholaminergic support which could be weaned at the end of the intervention.
One major complication (cardiac tamponade treated with surgical stitching of the left atrium) occurred in the patient group with ≤3 embolic lesions.
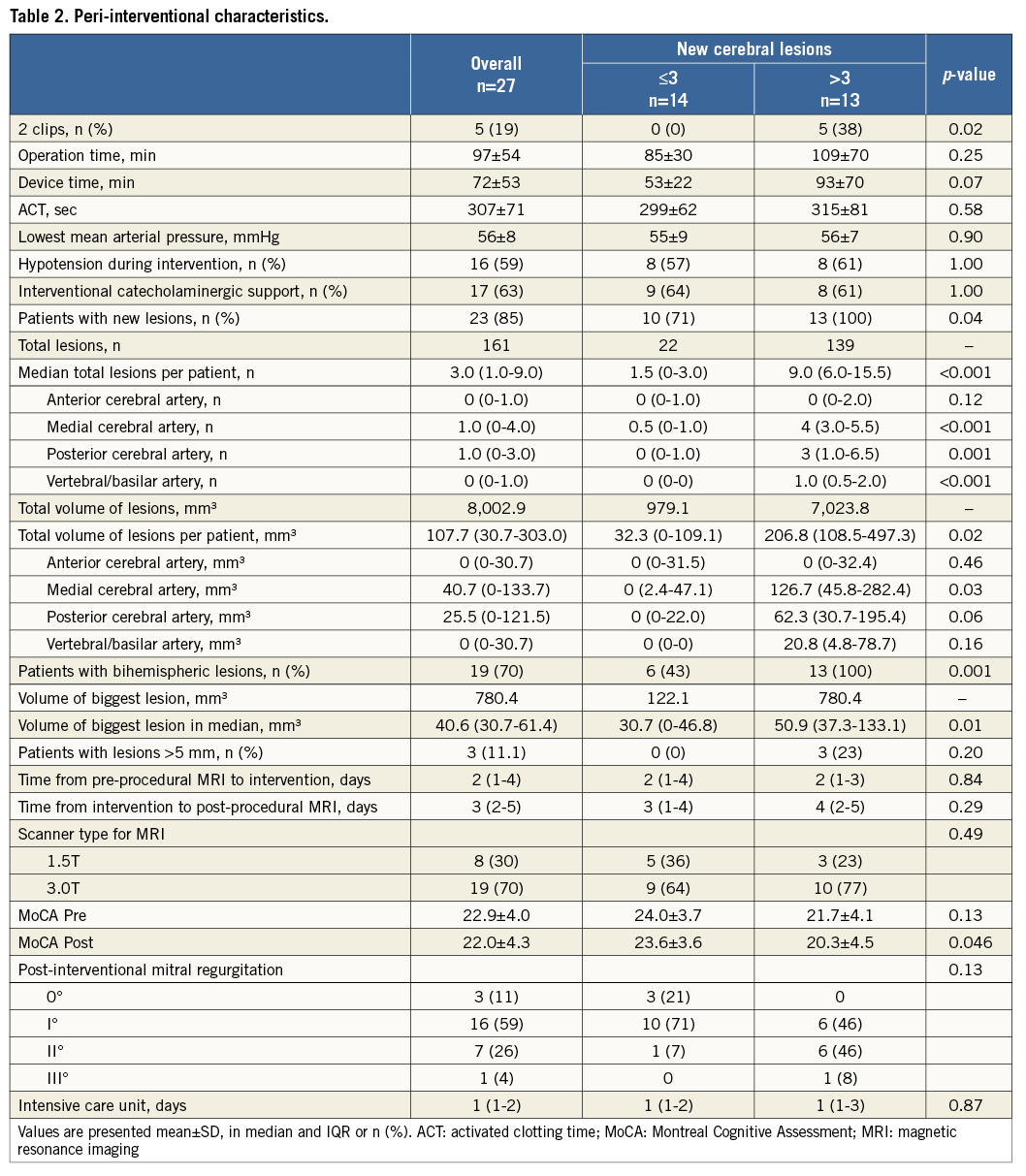
Additional peri-interventional characteristics are depicted in Table 1 and Table 2, respectively.
ECHOCARDIOGRAPHIC PARAMETERS
The severity of mitral regurgitation could be reduced from a median of three (IQR 3-3) to one (IQR 1-2); p<0.001. There were no significant differences in mitral regurgitation grade reduction between patients ≤3 and patients >3 new embolic lesions (median 2 [IQR 2-2.25] vs. median 1 [IQR 1-2], p=0.13, respectively).
BRAIN MRI
Preinterventional MRI was performed at a median of two days (IQR 1-4) before and post-interventional MRI at a median of three days (IQR 2-5) after the procedure, with no significant differences between the two groups ≤3 and >3 embolic lesions (p=0.84 and p=0.29, respectively).
MRI scans were obtained with 1.5T in eight (30%) and 3.0T in 19 (70%) patients, with no significant differences between groups with ≤3 and >3 embolic lesions (p=0.49).
All patients showed various degrees of microangiopathy, defined as diffuse affection of cerebral white matter in absence of other underlying pathologies, and no acute diffusion abnormalities at baseline MRI scans. Five patients had signs of previous infarction consistent with the history of prior stroke.
After intervention, a total of 161 (range 1 to 21) new lesions in DWI were found in 23 of 27 (85%) patients. In 20 (74%) patients, the post-interventional scan revealed multiple lesions dispersed in both hemispheres in a diffuse distribution pattern (Figure 2).
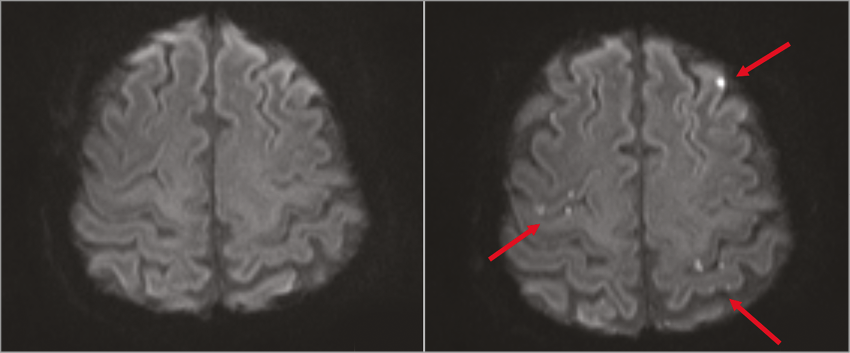
Figure 2. Pre- and post-interventional diffusion-weighted MRI. Diffusion-weighted MRI before (left) and after (right) the procedure showing multiple new ischaemic lesions dispersed over both hemispheres. MRI: magnetic resonance imaging
The volumetric assessment of lesions showed a median total volume (as a sum of every individual lesion) of 107.7 (IQR 30.7-303.0) mm³, whereas the biggest lesion was found to have a median extent of 40.6 (IQR 30.7-61.4) mm³.
The vascular distribution of the embolic lesions is set out in Figure 3. Detailed analyses of the peri-interventional MRI results are summarised in Table 2.
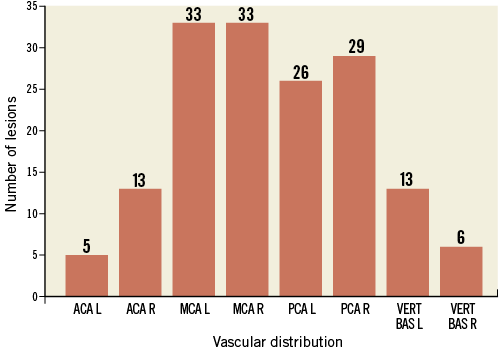
Figure 3. Number of embolic lesions and vascular distribution. Localisation of diffusion-weighted magnetic resonance imaging lesions after the procedure depicted as number of embolic events in each vascular territory in a total of 23 patients (85%). ACA: anterior cerebral artery; L: left; MCA: middle cerebral artery; PCA: posterior cerebral artery; R: right; VERT BAS: vertebro-basilar artery
PREDICTORS FOR CEREBRAL LESIONS
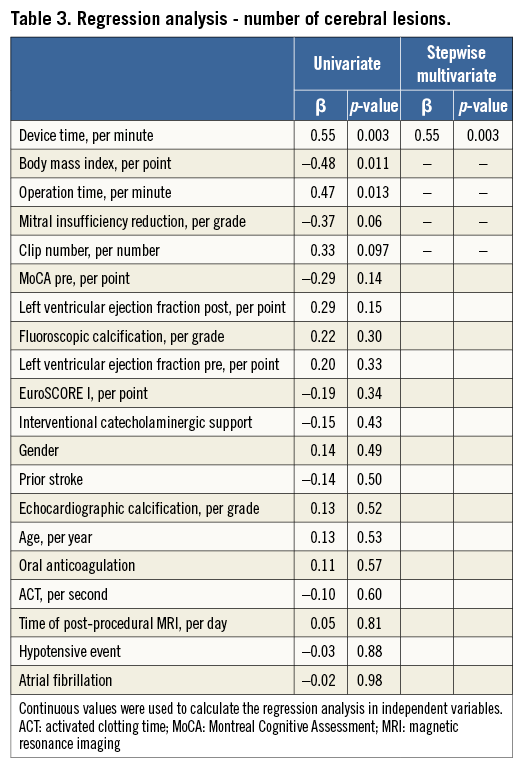
Univariate regression analysis for prediction of the number of new cerebral lesions revealed operation time, body mass index and device time as significant predictors. The numbers of clips needed and the degree of mitral insufficiency reduction showed a non-significant trend towards a higher number of lesions. On multivariate stepwise analysis, device time remained the only independent predictor for the number of post-procedural new lesions. The results for additional peri-interventional parameters are given in Table 3.
EVALUATION OF NEUROCOGNITIVE FUNCTION
By protocol, the neurological MoCA test was performed on the same day as the pre- and post-interventional MRI scans. No significant differences could be observed in the pre-interventional testing of cognitive function, but patients with >3 new post-interventional embolic lesions (n=13, 48%) had a lower post-interventional MoCA score in comparison to patients with ≤3 embolic lesions (23.6±3.6 vs. 20.3±4.5; p=0.046) (Table 2). Neither patients with ≤3 embolic lesions nor those with >3 embolic lesions showed a significant decline of post-interventional MoCA score in comparison to baseline score (24.0±3.7 vs. 23.6±3.6, p=0.54, respectively, and 21.7±4.1 vs. 20.3±4.5, p=0.15, respectively). At baseline and follow-up, none of the patients had any global or focal neurological deficit.
PREDICTORS FOR REDUCED POST-PROCEDURAL MoCA SCORE
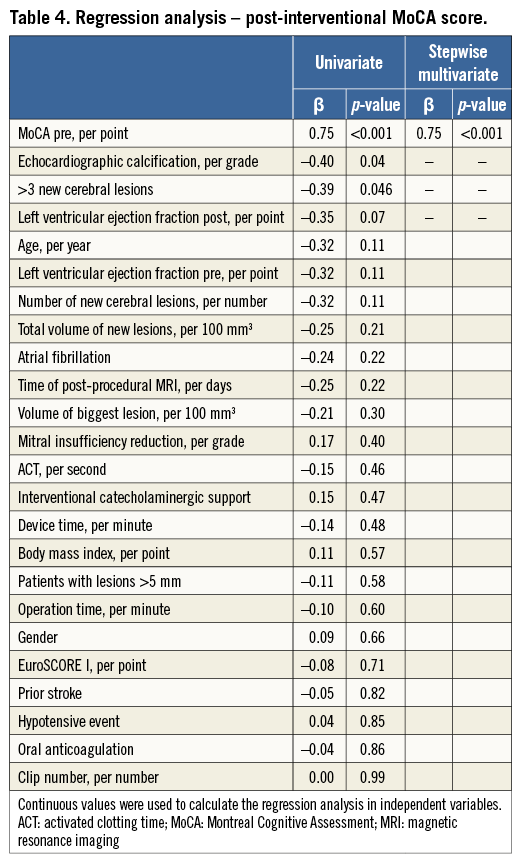
Valve calcification on echocardiography, pre-procedural MoCA score, new cerebral lesions larger than 5 mm and >3 new lesions were univariate predictors of lower post-procedural MoCA score. The post-interventional ejection fraction showed a trend towards a lower MoCA score. After multivariate testing only the pre-procedural MoCA score was an independent significant predictor of low post-procedural MoCA score (Table 4).
Discussion
To the best of our knowledge, this is the first study to provide insight into the risk of periprocedural cerebral ischaemic events in patients undergoing percutaneous mitral valve reconstruction. The MitraClip procedure was associated with a high rate of new cerebral lesions as detected by post-interventional DWI and in 70% of patients hyperintense lesions were multiple and in different vascular territories, strongly suggesting an embolic origin.
Multiple invasive diagnostic and interventional procedures bear the risk of periprocedural cerebral ischaemic events8,16. Although clinically silent, cerebral emboli have been described as being associated with functional neurological limitations and the development of dementia9-11. More frequent or extensive lesions in DWI seem to lead to a more rapid decline10,17 and speed up the development of neurocognitive impairment18,19.
In the current study, there were no significant differences in MoCA score between pre- and post-intervention. This was the case for the total population, the group of patients with ≤3 embolic lesions and the group of patients with >3 embolic lesions.
In multivariate testing, only the pre-procedural MoCA score was an independent significant predictor of low post-procedural MoCA score. There was no evidence that the number of lesions or total lesion size has an impact on the post-interventional MoCA score.
Potential explanations for the reason and origin of ischaemic lesions are: 1) dislodgement of micro-debris from chordae tendineae or the valve leaflets during advancement of the clip into the left ventricle and grasping attempts during the pullback manoeuvre; 2) device repositioning may lead to further embolisation, as reflected by device time as the only independent predictor for ischaemic lesions; 3) clot formation due to the thrombogenic nature of the guiding and delivery system or air embolism at any procedural step; 4) dislodgement of micro-debris in the left atrial appendage; and 5) formation of blood clots between the sheath and the dilator after transseptal puncture. In a recent study no cerebral events were described in 1,279 consecutive patients20. The Brockenbrough transseptal needle might shear off microparticles21. Intracardiac ultrasound could reveal thrombus formation 5-15 minutes after transseptal puncture in 1% or 9% depending on low- or high-dose heparin flushing of the catheters22.
In the current study, all catheters were flushed using heparin and ACT was >250 sec throughout the intervention in repeated measures every 30 minutes. Despite spontaneous contrast after transseptal puncture, device insertion and grasping attempts, no solid thrombus formation was observed in vigorous transoesophageal monitoring.
Hyperintense signals on DWI in correlation with hypointense signals in ADC are a surrogate parameter for cerebral embolisation8,23,24. In patients with transcatheter aortic valve implantation, a high risk for cerebral ischaemic lesions post procedure has been well documented25,26. However, the procedure is quite different in comparison to the MitraClip implantation. Embolic events during transcatheter aortic valve implantation are believed to be caused by embolisation of material from the site of implantation and the aorta. In contrast, the origin of embolic material during MitraClip implantation remains less clear. Interestingly, calcification of the mitral valve seemed not to have any impact on emboli occurrence.
Whereas a pilot study evaluating embolic protection devices in patients undergoing transcatheter aortic valve implantation yielded encouraging results27, the benefit of using such protection during MitraClip implantation remains unclear and should be addressed in the future.
Nonetheless, foci of diffusion restriction in MRI have also been associated with seizure, migraine, hypoglycaemia and especially hypotension. In general anaesthesia, cerebral perfusion is determined largely by systemic blood pressure, therefore low blood pressure or episodes of hypotension are associated with cerebral damage and stroke28. In this study, no correlation between blood pressure, episodes of hypotension, or catecholaminergic support and embolic lesions could be observed. However, it is of note that the MAP during the intervention was slightly lower in this study than recommended28. Although mechanisms other than embolism cannot be fully excluded, the missing boundary zone distribution pattern of the ischaemic lesions supports an embolic origin.
Limitations
Some limitations of this trial need to be mentioned. The limited number of patients impedes a robust multivariate statistical analysis identifying independent risk factors. The number of individual mitral valve leaflet grasp attempts during the intervention was not recorded. Through the use of different MRI equipment, it may be possible that some minor lesions were not detected in patients undergoing MRI examination with a 1.5T scanner. However, there were no differences in the number of detected lesions for the 1.5 or 3.0T scanners.
Clinical testing of neurological function was conducted with a standardised routine examination in the intensive care unit and global cognitive function was assessed using a brief test. Therefore, subtle changes may not have been detected. Due to the study design, the part of the procedure which might lead to the highest burden of microembolisation could not be examined. This information could be obtained in subsequent analyses using intrainterventional transcranial Doppler assessment of typical microembolic signals during the different interventional stages.
Conclusion
The MitraClip procedure results in new ischaemic cerebral lesions in the vast majority of patients. Preliminary data suggest that these lesions have no clinically significant impact on global cognitive function.
| Impact on daily practice Endovascular treatment options for valvular heart diseases bear the risk of cerebral embolism with catastrophic consequences. Clinically apparent periprocedural strokes after mitral valve repair using the MitraClip system have been reported in the range of 0-1%. In our cohort, comparison of pre- and post-interventional MRI showed that 23 of 27 patients (85.7%) had newly acquired clinically silent microembolic lesions, with in median 3 (interquartile range 1-9) new lesions per patient. Brief neurological testing detected no changes in neurocognitive function. New foci of restricted diffusion have been described as being associated with development of dementia and neurological limitation. In the future, protection devices and adaptations of the delivery catheter will reduce embolic burden during intervention. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

