
Abstract
Aims: The aim of this study was to assess the stroke rate after transcatheter mitral valve repair (TMVR) with the MitraClip, comparing it with surgical mitral valve repair (SMVR) and optimal medical treatment (OMT).
Methods and results: In December 2018, we systematically searched PubMed, Embase and Cochrane Controlled Register of Trials for studies comparing TMVR with SMVR and/or OMT for the treatment of severe mitral regurgitation. Random-effects and cumulative meta-analysis was performed. Ten studies were included (seven of TMVR versus SMVR and three of TMVR versus OMT), providing a total of 1,881 patients and 61 pooled strokes (16 in TMVR versus SMVR and 45 in TMVR versus OMT). There was no difference in stroke incidence between TMVR and SMVR (pooled OR 0.49 [0.17, 1.42], p=0.19). However, there was a trend towards a lower stroke risk in TMVR. For TMVR versus OMT, no difference in stroke rate was identified (pooled OR 1.09 [0.60, 1.97], p=0.79). Post-procedure de novo atrial fibrillation was more frequent in SMVR when compared with TMVR.
Conclusions: Despite both a low number of pooled stroke events and the failure to reach the pre-specified statistical significance, there was a trend for a lower post-procedure stroke rate in TMVR when compared with SMVR and a similar one between TMVR and OMT alone.
Introduction
It is well established that surgical mitral valve repair (SMVR) is the optimal treatment for severe mitral regurgitation (MR). However, a large proportion of patients is deemed unsuitable for surgical treatment, thus representing a striking unmet need in cardiovascular medicine1. So far, in patients with both primary and secondary symptomatic MR who are judged inoperable, only the edge-to-edge repair technique with the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) is globally used (class IIb indication in the most recent European Society of Cardiology guidelines)2.
The MitraClip is inspired by the surgical Alfieri technique, which creates a double orifice mitral area3. As a result, the physiology of diastolic transmitral flow is modified, leading to some restriction in left ventricle filling. Thus, this haemodynamic profile could result in blood stasis, an increased risk of left atrial thrombosis and, consequently, the risk of thromboembolic events4,5,6,7. Additionally, atrial fibrillation (AF) complicates the course of MR and is itself a risk factor for stroke and peripheral embolic events8. Although this rationale might seem insufficient to offset cardiac surgery stroke risk, it may embody the recommendation for anticoagulation, particularly with vitamin K antagonists, which are the only oral anticoagulants indicated in both mitral prosthesis and mitral stenosis9. Despite this, no strict peri- and post-MitraClip procedure antithrombotic therapies have been defined so far; distinct protocols are currently being applied10,11.
Systematic reviews support the long-term safety of transcatheter mitral valve repair (TMVR) with the MitraClip for degenerative and functional MR plus the durability of MR reduction12,13. Recent meta-analyses have demonstrated that, compared with conservative treatment alone, TMVR is associated with a significant relative risk reduction of death from any cause and heart failure in high-risk patients with left ventricular dysfunction14,15. However, none of these reviews specifically analysed stroke incidence. In 2018, the COAPT trial16 reinforced the increased safety of the edge-to-edge repair technique compared to optimal medical treatment (OMT), while the MITRA-FR trial17 reported no significant difference in adverse effects between the two groups.
We aimed to carry out a systematic review of the published literature on the comparison between TMVR with the MitraClip device and both SMVR and OMT groups of patients, analysing stroke incidence among these therapeutic options for MR.
Methods
PROTOCOL AND REGISTRATION
This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A registration (CRD42018117614) in the PROSPERO database was made at inception.
LITERATURE SEARCH
Based on the PRISMA statement, we systematically searched PubMed, Embase, and Cochrane Controlled Register of Trials (CENTRAL) in December 2018, for both interventional and observational studies comparing TMVR with SMVR and/or OMT for the treatment of severe MR. The search was limited by language (English, French, Portuguese, or Spanish) and type of subjects (human). No date publication limits were imposed. Supplementary Figure 1 shows the search strategy of this review. Additional data were collected from randomised controlled trial (RCT) protocols. Different publications from the same patient cohorts were considered as a single study for the purpose of this review.
ELIGIBILITY CRITERIA
The following criteria were used to define study eligibility: 1) RCTs or observational studies comparing the MitraClip procedure with mitral valve surgery and/or OMT; 2) participants with severe MR; and 3) information on stroke incidence after procedure. We excluded series with fewer than 20 patients or without full-text article publications.
PRIMARY AND SECONDARY OUTCOMES
Primary outcomes were early (<30 days) and late (>30 days) post-procedural stroke rate. Secondary endpoints were de novo AF and bleeding events.
DATA COLLECTION AND MANAGEMENT
Two authors (P. Barros da Silva and J.P. Sousa) systematically screened the titles and abstracts of publications retrieved using the search strategy in order to select studies which met the inclusion criteria outlined above. The full texts of the eligible studies were, again, independently assessed for eligibility by the two review team members. Any disagreement between them over the eligibility of particular studies was resolved through discussion and involvement of a third author (R. Teixeira), when necessary. Data extraction concerned the study population, main demographics and baseline characteristics, interventions, and the outcomes described above. We analysed studies with multiple sequenced publications, ensuring no duplication of results and the collection of the most recent data.
Some studies did not break down information on early (<30 days) versus late (>30 days) post-procedural stroke incidence. For this reason, and to increase statistical power, we joined both outcomes, creating an all-stroke post-procedural rate. Post-procedural stroke incidence included both ischaemic and haemorrhagic cerebrovascular events, due to the lack of separate outcomes in the majority of studies.
To compare the incidence of bleeding events, we included major bleeding and need for blood transfusions (>1 unit) in postoperative care.
RISK OF BIAS ASSESSMENT
Two authors (P. Barros da Silva, J.P. Sousa) independently assessed the risk of bias of the included articles, following the Cochrane Collaboration’s “Risk of bias” tool for RCTs and the Newcastle-Ottawa Scale for observational studies. RCTs were assessed as “low”, “high” or “unclear” risk for the following biases: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. None of the included studies had a blinding strategy, which resulted in a high risk for performance and detection bias. This assessment was expected, because the MitraClip device is visible on imaging studies, most studies were retrospective, and in the two RCTs on TMVR versus OMT there was no sham procedure. The quality assessment for each study is presented in the “risk of bias summary” (Figure 1) and Newcastle-Ottawa Scale summary (Figure 2).
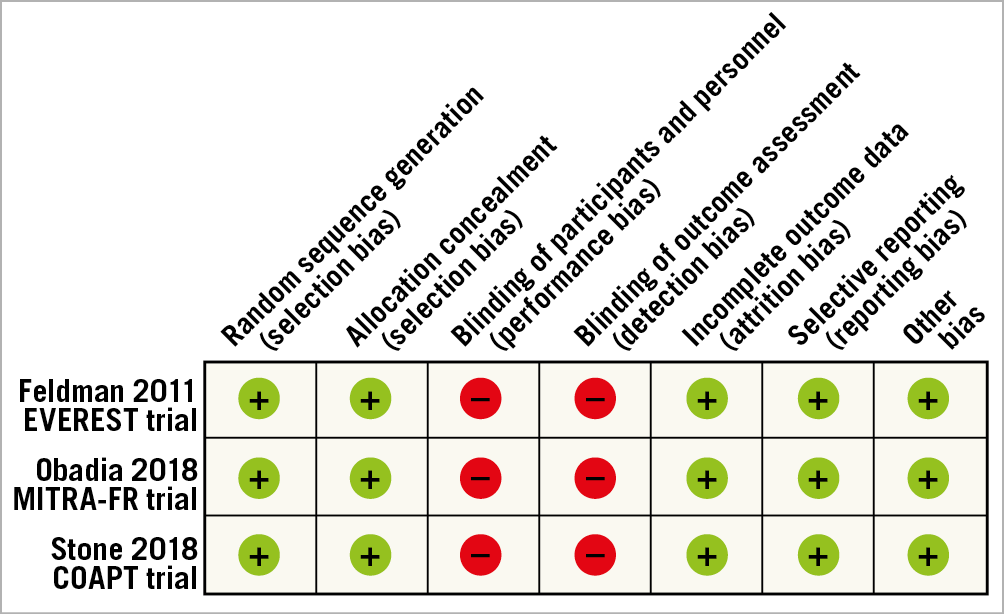
Figure 1. Risk of bias summary.
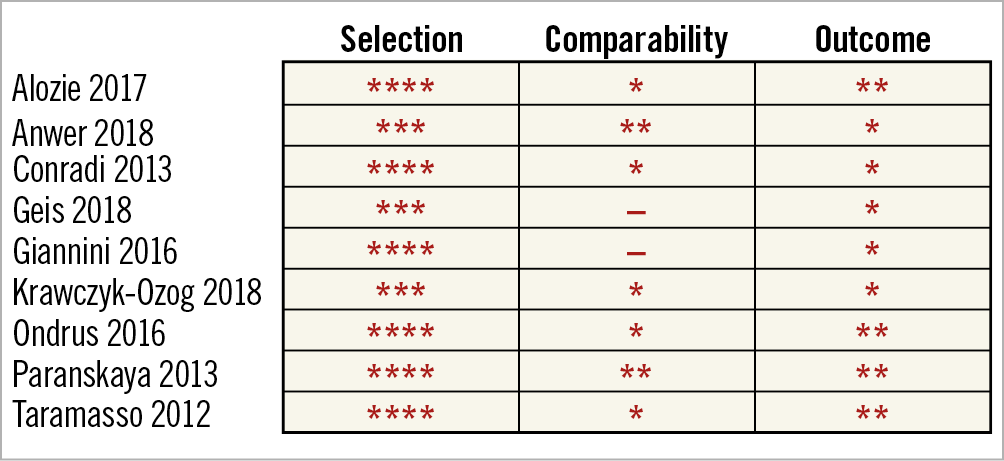
Figure 2. Newcastle-Ottawa Scale summary.
STATISTICAL ANALYSIS
We pooled dichotomous non-adjusted data using odds ratios (OR) to describe effect sizes using the Mantel-Haenszel procedure in a random effects model. We also performed a continuity correction for the individual odds ratios and the overall measure, which considers adding the quantity 0.5 to all cells whenever relative effect measures are undefined due to the presence of zeros. Study heterogeneity was evaluated by funnel plots while publication bias was evaluated using Egger’s test and both Galbraith and normalised Galbraith plots. To evaluate temporal trends on stroke incidence, a cumulative meta-analysis was performed according to date of publication, following a usual meta-analysis. In the cumulative analysis, studies were successively added by year of publication and the 95% confidence intervals (CIs) and overall cumulative odds ratios were recalculated, enabling us to evaluate the outcome evolution over time. The impact of both age and left ventricular ejection fraction (LVEF) on stroke incidence between TMVR and SMVR was analysed by meta-regression based on the mixed-effects model.
The mean effect was considered significant if its 95% CI did not include zero. Heterogeneity was assessed using the I² statistic and assumed to be relevant if it exceeded 50%.
The cumulative meta-analysis and the meta-regression was performed using R software through R Studio, version 1.1.463 (R Foundation for Statistical Computing, Vienna, Austria), and the traditional meta-analysis using RevMan 5.3 (Cochrane Community, London, UK).
Results
SEARCH RESULTS
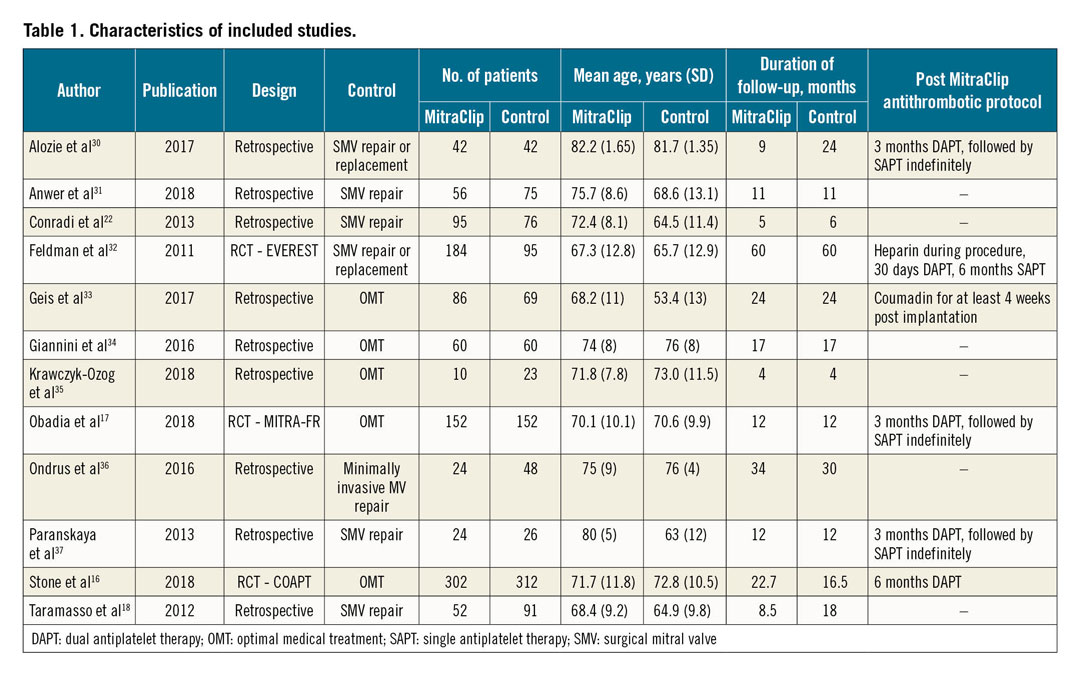
The literature search identified 1,447 articles. Two cited RCTs (MITRA-FR and COAPT) were added. After duplication removal, we excluded a total of 1,194 publications based on title and abstract evaluation, study type (RCTs or observational studies comparing the MitraClip procedure with SMVR and/or OMT) and study population (participants with severe MR). The full text of the remaining 47 studies was then screened, leading to the exclusion of 35 publications: three studies only included outcomes of MitraClip populations, one study only reported stroke rates on the mitral valve surgical arm, two full texts could not be accessed, eight did not specifically refer to post-procedure stroke incidence, 12 were conference abstracts or RCT design studies, three used data from the EVEREST trial for a different analysis and three RCTs did not yet include any published results; the study by Taramasso et al was chosen among four studies based on the same institutional population, as it included a wider number of participants18,19,20,21. Finally, 12 publications met all the inclusion criteria for the qualitative review; 10 of these were suitable for the quantitative synthesis with meta-analysis (Figure 3). Seven studies compared TMVR versus SMVR and three TMVR versus OMT, providing a total of 1,881 patients and 61 pooled strokes (16 in TMVR versus SMVR and 45 in TMVR versus OMT). Baseline characteristics of the included studies are shown in Table 1.
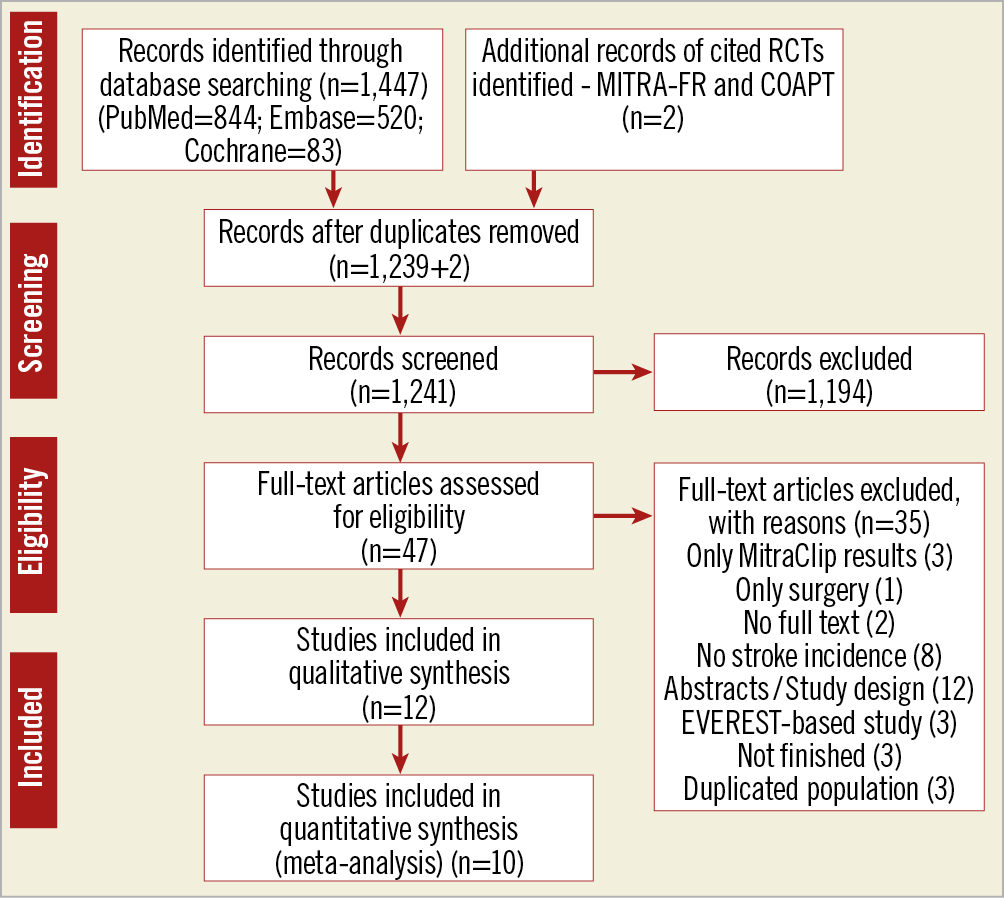
Figure 3. Flow diagram of literature search.
MITRACLIP VERSUS SURGERY
We identified seven studies comparing TMVR using the MitraClip with surgical repair/replacement. TMVR patients were older and had higher surgical risk scores than SMVR patients. The groups were homogeneous regarding previous AF rate (pooled OR 1.45 [0.82-2.55]) (Figure 4), whereas post-procedure de novo AF was more frequent in SMVR compared with TMVR (pooled OR 0.20 [0.06-0.7]) (Figure 5) in the four studies that reported data on 30-day post-procedural AF.
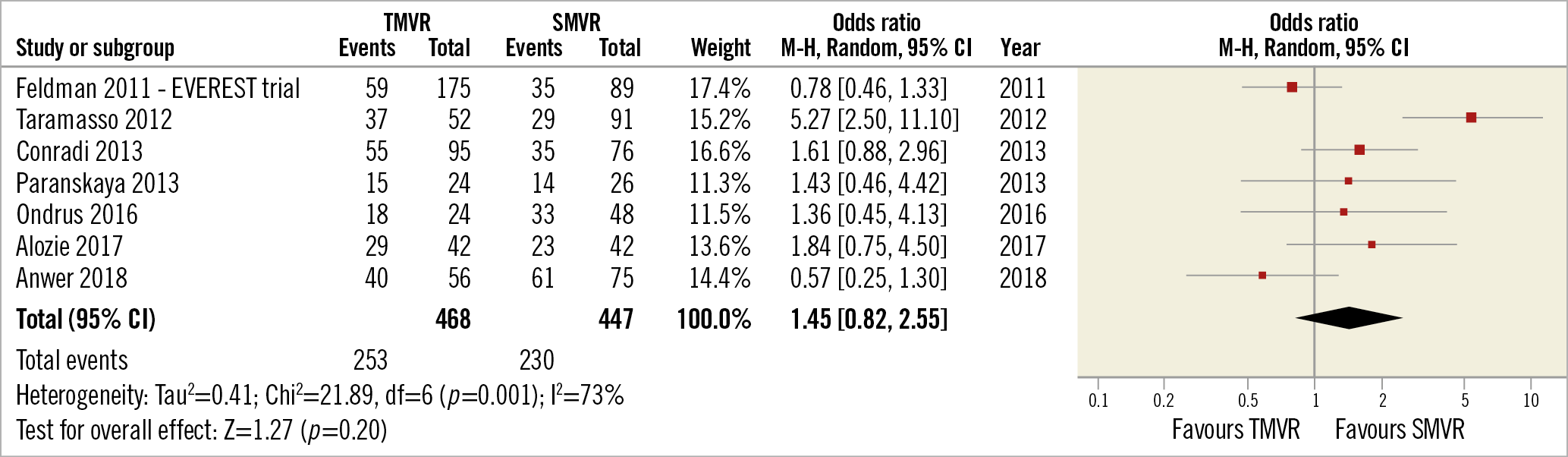
Figure 4. TMVR vs SMVR – previous atrial fibrillation.
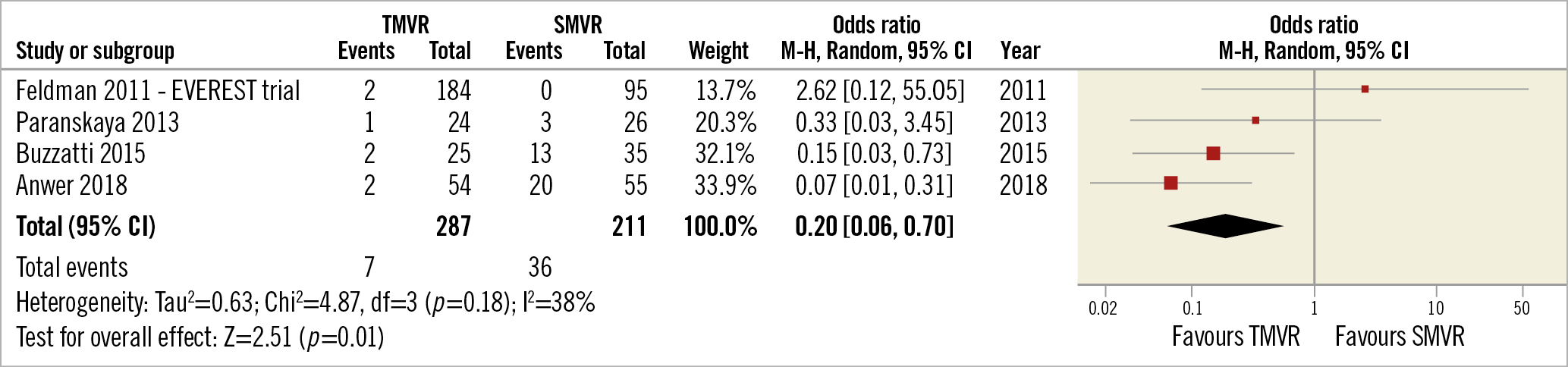
Figure 5. TMVR vs SMVR – de novo atrial fibrillation.
There was no significant difference in stroke incidence between TMVR and SMVR (pooled OR 0.49 [0.17, 1.42], p=0.19, I²= 0%) (Figure 6, Figure 7). However, by approximating this OR’s confidence interval distribution to a standard normal one, we found that the interval between 0.17 and 1 is responsible for 77.5% of the distribution. In other words, obtaining a definitive OR favouring TMVR is 3.435 times more likely than obtaining a definitive OR favouring SMVR.
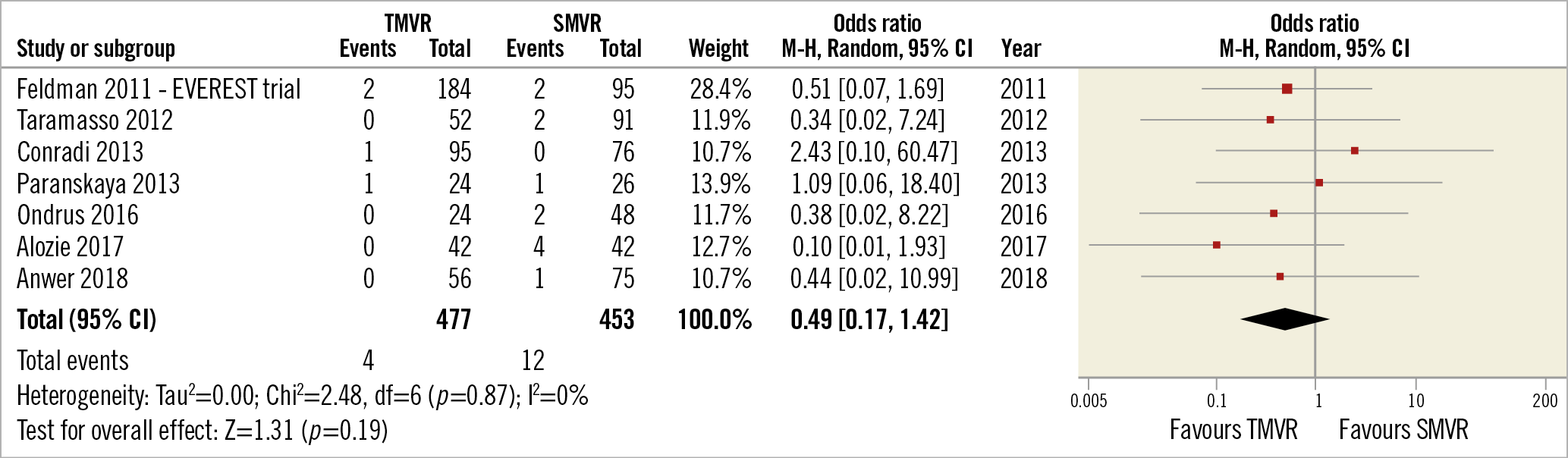
Figure 6. TMVR vs SMVR – all stroke incidence.
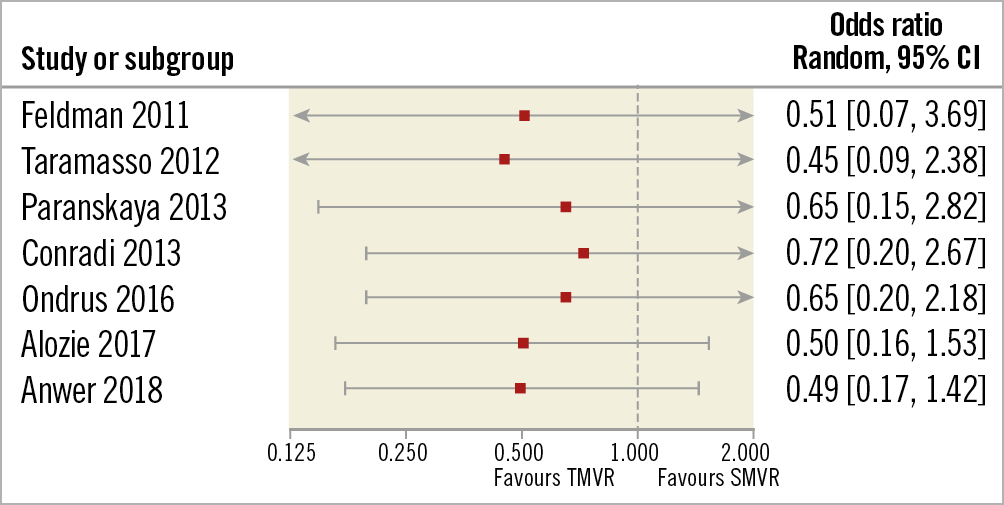
Figure 7. TMVR vs SMVR – all stroke incidence, cumulative meta-analysis.
The studies were homogeneous. No selection or publication bias was identified by funnel plots, which was confirmed by the Galbraith or the normalised Galbraith plots (Supplementary Figure 2-Supplementary Figure 4). Moreover, the Egger test confirmed that the normalised effects were independent from the precision (b=0.38, p=0.82, t value=0.24; R2=0.013) (Supplementary Figure 5). Also, the Begg and Mazumdar test confirmed that the effects were not related to their variance (Kendall’s tau=0.14; p=0.77).
Meta-regression for age and LVEF had small effect sizes with no statistically significant p-values (respectively Z=–0.61, p=0.54 and Z=0.038, p=0.97).
Bleeding events were less frequent in TMVR compared to SMVR (pooled OR 0.25 [0.11, 0.56], p<0.05, I²=33%) (Figure 8).
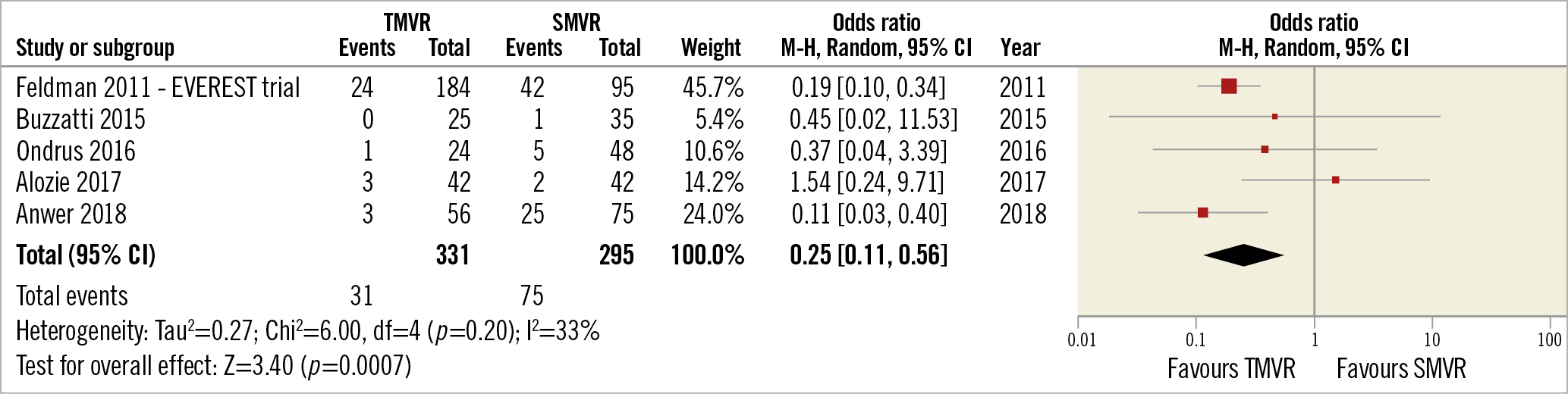
Figure 8. TMVR vs SMVR – bleeding events.
MITRACLIP VERSUS OMT
There were five studies comparing TMVR using the MitraClip plus OMT with OMT alone. However, only three of these provided data on stroke incidence during follow-up in both the TMVR and OMT groups and these were used for meta-analysis. No difference in stroke rate was identified (pooled OR 1.09 [0.60, 1.97], p=0.79, I²=0%) (Figure 9).
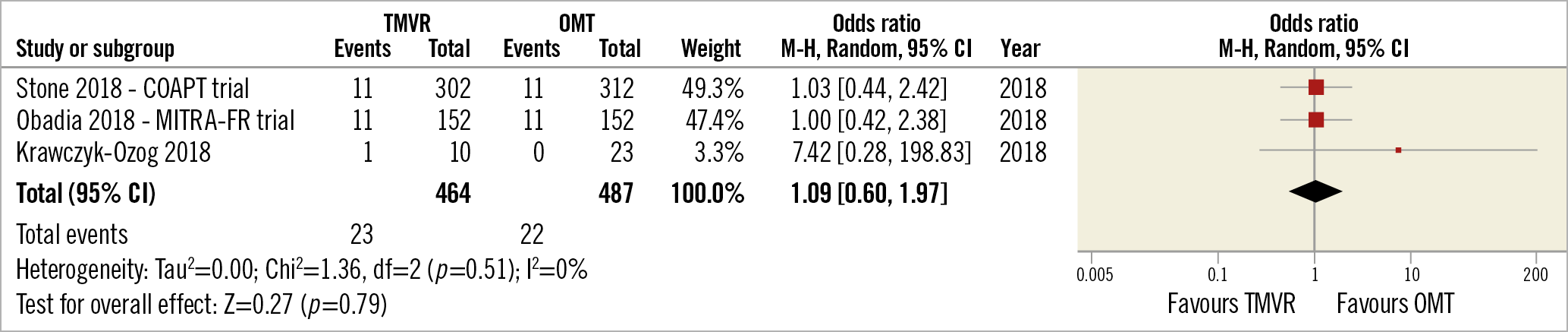
Figure 9. TMVR vs OMT – all stroke incidence.
Discussion
According to our meta-analysis in the context of MR treatment, (i) the pooled stroke rate after TMVR, SMVR and OMT for MR patients had a low number of events, (ii) there was a trend towards a lower stroke rate for patients submitted to TMVR compared to SMVR, (iii) there was a similar stroke rate for patients treated with TMVR when compared to patients allocated to OMT, and (iv) post-procedure AF was more frequent after SMVR when compared to TMVR.
The decision to deny surgery for patients with MR is mostly based on impaired LVEF, age, and comorbidity1. For this reason, MitraClip patients are theoretically at increased risk for AF and stroke. However, our results do not confirm that hypothesis. In addition, they support the most recent systematic review that reported similar survival for TMVR with MitraClip and surgery, despite patients’ higher risk profiles in the TMVR group23. However, to the best of our knowledge, this is the first and only meta-analysis specifically to examine stroke and de novo AF rates after mitral valve repair with the MitraClip, comparing it with both surgery and OMT.
OMT
In patients who are not candidates for surgery, the option between TMVR with the MitraClip and OMT alone is still debated: the MITRA-FR trial17 showed that both treatments were similar regarding adverse effects, but the results of the COAPT trial16 favoured the MitraClip for the same outcome. In terms of stroke incidence, our analysis showed that the stroke rate post TMVR with the MitraClip was similar to that of patients managed with OMT alone. This finding is unexpected, as, for instance, transseptal puncture for catheter ablation poses a risk of paradoxical embolism, which has already been described for catheter ablation of arrhythmia24. Selecting patients for percutaneous treatment with optimal risk–benefit balance is still subject to ongoing research. Hopefully, the RESHAPE-HF trial may solve the present controversy regarding MitraClip safety compared to conservative treatment, allowing a more sustained clinical decision25.
MITRAL VALVE SURGERY
Even though our meta-analyses disclosed no statistically significant difference in stroke incidence between TMVR and SMVR, a trend towards a lower stroke risk in post-TMVR patients was identified. For instance, the cumulative meta-analysis demonstrated this finding at all times26. Cumulative meta-analysis is still not a widely used statistical tool, but this chronological combination of studies has been proposed as a valuable method to decide when to stop ongoing trials or to adopt or reject an investigated treatment by different authors27,28.
A 2018 meta-analysis reported a negative effect of previous AF in the TMVR one-year survival rate; however, it did not address specific post-procedure adverse events or de novo AF13. On the other hand, a recent retrospective study comparing TMVR with MitraClip in patients with and without previous AF reported no significant difference in stroke incidence during follow-up29. The lower stroke rate for TMVR compared with SMVR might therefore be related to the lower incidence of post-procedure de novo AF in TMVR.
ANTITHROMBOTIC THERAPY
Only six of the 12 studies described post-procedure antithrombotic strategy, and only three were similar, hindering a comprehensive analysis of the protocols used. Antithrombotic therapy has never been formally evaluated in terms of outcome events in this setting. Both direct oral anticoagulants and vitamin K antagonists are currently used. However, considering their biomechanical similarity, there is the possibility of a contraindication to direct oral anticoagulants in MitraClip, as in mitral stenosis and mitral prostheses9. Nevertheless, the similar stroke incidence for TMVR and OMT indicates no concern regarding the use of direct oral anticoagulants after the MitraClip procedure.
FUTURE PERSPECTIVES
The call for a specific antithrombotic strategy is justified not only by the high prevalence of AF in TMVR with MitraClip candidates but also by the increased risk of left atrial thrombus formation after the procedure, caused by acute reduction of MR and changes in haemodynamics within the left atrium4,5,6,7.
Our findings suggest the need for RCTs on different post-MitraClip procedure antithrombotic treatments, in order to define a strict protocol, particularly for patients with AF. This is of major interest, because AF is highly prevalent in TMVR with MitraClip candidates, and the choice of antithrombotic treatment has a high impact on both quality of life and health costs.
Limitations
The most important limitation of our meta-analysis is the paucity of RCTs on this issue. In fact, the majority of studies included were observational and not randomised, increasing the risk of bias and therefore limiting the strength of our results. Furthermore, for our primary outcome there were only 16 events among the 930 patients included in the comparison between TMVR and SMVR, which may have single-handedly reduced the odds of unveiling a significant difference in stroke rates. We tried to overcome this limitation by applying a continuity correction and a cumulative meta-analysis28. One could argue that the low event rate is the justification for the statistical homogeneity found (I2=0%), despite numerical heterogeneity (odds ratios ranging from 0.1 to 2.43), that enabled the performance of a cumulative meta-analysis. However, by combining study events and sample sizes, cumulative meta-analysis may improve the statistical power as similar results are combined cumulatively. This method reduces the problem caused by the low event rate and especially by the presence of zero values in study groups. Regarding pooled stroke, data were not available for fatal versus non-fatal stroke, haemorragic stroke or transient ischaemic attack.
Conclusions
Although there was a low number of pooled events, our methodology showed a trend towards a lower post-procedure stroke rate for TMVR when compared with SMVR, possibly related to a lower incidence of de novo AF found in the percutaneous group. For the same outcome, rates were similar between TMVR and OMT alone. A clinical trial comparing MitraClip patients with and without previous AF, with and without anticoagulant therapy in the first group, is still needed to resolve this dilemma.
|
Impact on daily practice Our findings may prove insightful for future recommendations regarding the conundrum of the best antithrombotic strategy, particularly for patients with AF. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

