Abstract
Aims: We sought to determine the feasibility of conducting percutaneous coronary intervention (PCI) in high-risk acute coronary syndrome (ACS) patients utilising the REG1 system consisting of pegnivacogin, an aptameric factor IXa inhibitor, and its controlling agent anivamersen.
Methods and results: In RADAR, ACS patients were randomised to pegnivacogin 1 mg/kg with 25%, 50%, 75%, or 100% anivamersen reversal or unfractionated heparin. Of the 640 patients randomised, 388 (61%) underwent PCI. Major modified ACUITY 30-day bleeding rates were 18% (25% reversal), 12% (50% reversal), 9% (75% reversal), and 7% (100% reversal), compared with 11% with heparin. The corresponding total bleeding rates were 68%, 39%, 35%, 34%, and 38% (heparin). Ischaemic events were less frequent in those receiving pegnivacogin versus heparin (4.4% vs. 7.3%, p=0.3). Thirty-day urgent TVR (1.1% vs. 0.9%, p=1.0), myocardial infarction (4.0% vs. 6.4%, p=0.3), and angiographic complication (11.2% and 10.8%, p=0.9) rates were similar with pegnivacogin and heparin. There were no incidences of clot formation on guidewires or catheters.
Conclusions: High-level factor IXa inhibition in ACS patients undergoing PCI, with at least 50% reversal, has a favourable bleeding profile and appears effective at suppressing ischaemic events and thrombotic complications. Larger phase trials in PCI are warranted. Clinical Trials Registration: ClinicalTrials.gov NCT00932100.
Introduction
Anticoagulant therapy is vital to the safe conduct of percutaneous coronary interventions (PCI); however, attempts to increase effectiveness have been limited by bleeding1. While the impact of ischaemic events, including peri-PCI infarction, has long been appreciated2, the importance of bleeding on long-term outcomes3,4 has also received increasing attention. One approach to achieving effective anticoagulation while mitigating bleeding is to use an anticoagulant regimen that can be actively reversed, permitting anticoagulant therapy to be tailored to the clinical circumstances and changing needs of an individual patient.
The REG1 anticoagulation system consists of pegnivacogin, a single-stranded RNA factor IXa inhibitor, and its reversal agent, anivamersen, which binds to and inactivates pegnivacogin via Watson-Crick base pairing5, and represents the first use of a factor IXa inhibitor in this clinical context.
RADAR (a randomised, partially blinded, multicentre, active-controlled, dose-ranging study assessing the safety, efficacy, and pharmacodynamics of the REG1 anticoagulation system in patients with acute coronary syndromes) suggested the feasibility of using pegnivacogin 1 mg/kg with at least 50% anivamersen reversal in patients with acute coronary syndromes (ACS) undergoing catheterisation6. However, the safety of conducting PCI with this anticoagulation strategy has not been thoroughly investigated. A phase 2a study demonstrated the safe conduct of low-risk PCI in a limited number of cases7; however, the RADAR study represented the first significant experience with factor IXa inhibition using REG1 in patients undergoing PCI6.
The objectives of RADAR-PCI are to determine the safety of pegnivacogin/REG1 compared with heparin on bleeding, procedural complications, and ischaemic events in patients with ACS undergoing PCI.
Methods
STUDY POPULATION AND DESIGN
The design8 and overall results of RADAR have been published6. RADAR was a phase 2b, international, adaptive-design, partially blinded, dose-ranging clinical trial. Patients were enrolled at 67 centres in the United States, Poland, Germany, Canada, France, and The Netherlands between September 2009 and November 2010. The protocol was approved by institutional review boards or ethics committees at each institution and all patients provided written informed consent prior to participation.
ELIGIBILITY CRITERIA
Eligible patients were required to have a minimum of 10 minutes of ischaemic symptoms within 72 hours of enrolment associated with (1) dynamic ST-segment changes, (2) elevated troponin I or T or CK(-MB), or (3) a history of coronary artery disease by angiography. In addition, patients had to have planned cardiac catheterisation via femoral arterial access within 24 hours of randomisation, and underwent PCI at the discretion of the investigator. Major exclusion criteria included: (1) ST-segment elevation myocardial infarction (MI); (2) weight >120 kg; (3) prior use of low molecular weight heparin within three days or of warfarin, bivalirudin, fibrinolytic agents, or glycoprotein IIb/IIIa inhibitors within seven days; (4) planned use of >7 Fr sheaths, intra-aortic balloon pump, or other support devices; or (5) an inability to take aspirin or a thienopyridine.
RANDOMISATION AND STUDY INTERVENTION
Using a central interactive voice response system, patients were randomised 3:1 to open-label pegnivacogin with sheath removal 10 minutes post procedure, or heparin with sheath removal per standard of care. Aspirin and thienopyridine pretreatment were recommended. Glycoprotein IIb/IIIa inhibitors were recommended in patients assigned to heparin. A primary goal of RADAR was to determine the degree of anivamersen-mediated reversal required for safe sheath removal post femoral cardiac catheterisation. Patients randomised to pegnivacogin received 1 mg/kg over one minute intravenously pre-procedure, and were randomised, in a 2:1:1:2 ratio, to a blinded post-procedure dose of anivamersen of 0.075, 0.2, 0.4, or 1 mg/kg to achieve 25%, 50%, 75%, or 100% reversal of pegnivacogin9,10. Additional anivamersen (1 mg/kg) could be administered if haemostasis was not achieved after 20 minutes. Manual sheath removal was performed using a standardised procedure either immediately post procedure/reversal (REG1 patients) or according to local practice (heparin patients), with guidance to sites to remove the sheath when anticoagulation status in the heparin arm allowed safe sheath removal. Vascular closure devices were used at the same time points.
ENDPOINT DEFINITIONS
The composite ischaemic endpoint was death, non-fatal MI, urgent target vessel revascularisation, or recurrent ischaemia in the target vessel distribution through 30 days. Specific procedural complications, including abrupt or threatened coronary artery closure, distal embolisation, clot formation on catheters or wires, no reflow, or any other thrombotic complication, as well as the presence of thrombus on initial angiography, were recorded at the time of catheterisation and specifically queried at the time of PCI. Bleeding was determined using a modified ACUITY scale8. All bleeding and ischaemic events were monitored through 30 days and were adjudicated using original source documents by an independent clinical events committee blinded to study treatment.
STATISTICAL METHODS
Analyses were performed at the Duke Clinical Research Institute (Durham, NC, USA) with full access to the trial database. All analyses were performed on an intention-to-treat basis. Baseline, treatment, and complication characteristics are described as frequencies and percentages for categorical variables and median (25th, 75th percentiles), median (range), or mean (standard deviation) for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were provided for the ischaemic event composite (comparing REG1 with heparin) and for bleeding events (comparing REG1-100% reversal with heparin and REG1-100% reversal with REG1-25% reversal). Statistical testing for these comparisons was performed using Fisher’s mid-p test. Comparisons of demographic data, treatment characteristics and angiographic complications used the likelihood ratio chi-square test or Fisher’s exact test, as appropriate, for categorical variables and Wilcoxon rank-sum test for continuous variables. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
BASELINE CLINICAL AND DEMOGRAPHIC CHARACTERISTICS
Of 640 patients enrolled in RADAR, 388 (61%) underwent PCI (Figure 1). The baseline characteristics were well balanced between treatment arms (Table 1) and reflected those of the overall RADAR population. Enrolment in the pegnivacogin with 25% reversal arm was stopped after the first data and safety monitoring board meeting per protocol due to excess bleeding6.
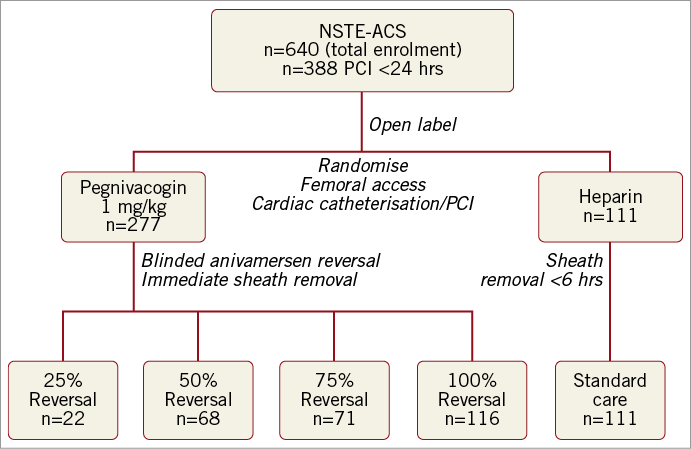
Figure 1. RADAR-PCI study diagram.
PROCEDURAL CHARACTERISTICS
Among patients undergoing PCI, 99.3% of patients in the pegnivacogin arms and all patients in the heparin arm received assigned therapy (Table 2). PCI was performed a median of 10.1 (25th, 75th percentile: 8.4, 11.2) and 9.8 (7.0, 10.9) hours after randomisation in REG1 and heparin patients, respectively. Use of thienopyridines was high and similar in the pegnivacogin and heparin arms, with prasugrel utilised in a minority of patients (Table 2) and more frequently in the heparin arm. Treatment arms were similar in numbers and types of lesions treated as well as other procedural characteristics.
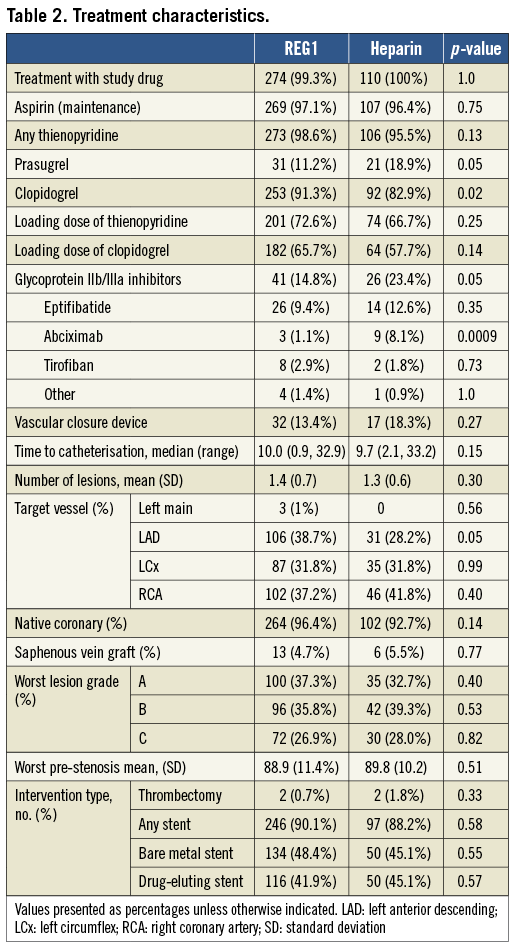
Use of glycoprotein IIb/IIIa inhibitors was low in both arms, but modestly higher in heparin patients (23.4 vs. 14.8%) where its use was recommended. Glycoprotein IIb/IIIa inhibitors were started prior to catheterisation in one patient in each arm, and after the initiation of catheterisation in 37 (13.6%) and 24 (21.6%) patients randomised to pegnivacogin and heparin, respectively. Among patients assigned to heparin, mean±standard deviation and median (25th, 75th) local activated clotting time values were 216±181 and 193 (143, 999) pre-procedure and 219±5 and 223 (183, 255) post-procedure, respectively.
Outcomes
PROCEDURAL COMPLICATIONS
The incidence of angiographic complications (Table 3), including mechanical and thrombotic issues, was similar in REG1 and heparin-treated patients (11.2 vs. 10.8%, p=0.9). Thrombus was noted on initial angiography in 6.9% and 7.2% of REG1 and heparin subjects, respectively. Thrombus formation during index PCI was noted in three (1.1%) REG1 patients and one (0.9%) heparin patient. No periprocedural thrombus formation on guidewires or catheters was observed in any patient, and no REG1 patients required transition from pegnivacogin to heparin. The rates of other thrombotic or potentially thrombotic complications (abrupt closure, distal embolisation, no reflow) were low. Periprocedural MI (occurring within 24 hours of PCI) occurred in 2.2% (REG1) and 4.5% (heparin) of subjects.
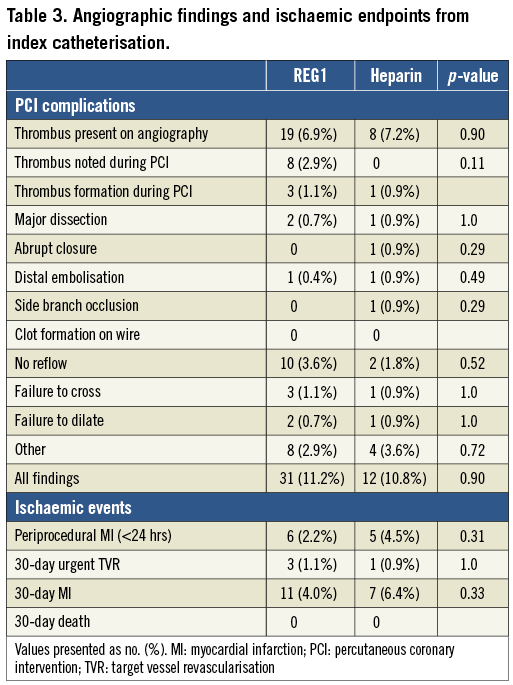
Thirty-day ischaemic events were similar in the two treatment arms. Notably, there were no deaths, MI occurred in 4.0% and 6.4% of REG1 and heparin patients, and urgent target vessel revascularisation through 30 days was rare and similar in both arms (1.1% [REG1] vs. 0.9% [heparin]); this was not related to the degree of pegnivacogin reversal, with one event in the 25%, 75%, and 100% reversal groups. The composite of 30-day death, non-fatal MI, urgent target vessel revascularisation, or recurrent ischaemia in the target vessel distribution was numerically but not statistically lower in patients assigned to pegnivacogin compared with heparin (Figure 2).
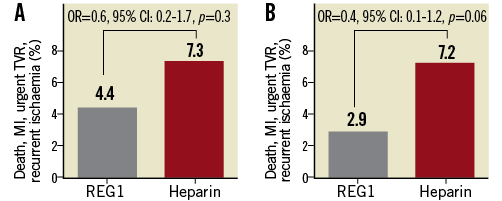
Figure 2. Incidence of the composite ischaemic endpoint (death, non-fatal MI, urgent target vessel revascularisation, or recurrent ischaemia in the target vessel distribution). A) Incidence at 30 days. B) Incidence through hospital discharge.
BLEEDING
Bleeding was substantial in the 25% reversal arm prior to discontinuation of enrolment (Figure 3), but similar among the other pegnivacogin arms and in patients randomised to heparin. There was a stepwise trend between total and major modified ACUITY bleeding with degree of reversal. Additional open-label anivamersen was required in 27.3%, 4.5%, 4.3%, and 2.6% in the 25%, 50%, 75%, and 100% reversal arms, respectively, mirroring rates of major bleeding. Although RADAR was not powered to compare bleeding rates statistically in this cohort, there was a lower rate of total bleeding at 30 days with REG1 and 100% reversal compared with REG1 and 25% reversal (p=0.003) (Figure 3). Excluding major bleeding events defined solely on the basis of the presence of a 5 cm haematoma, a component of ACUITY but not many other bleeding scales, resulted in similar results (bleeding in 13.6%, 4.5%, 4.2%, and 3.5% in the 25%, 50%, 75%, and 100% reversal arms, respectively, and 5.5% in the heparin arm).
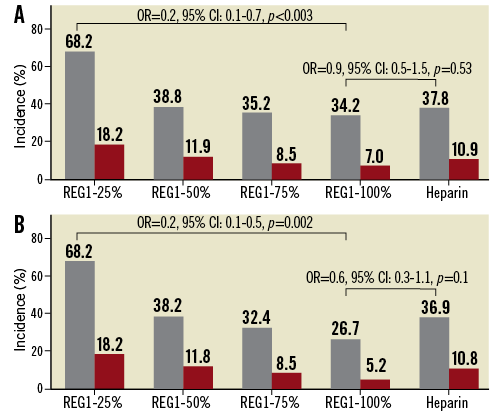
Figure 3. The number and incidence of total and major modified ACUITY-defined bleeding. A) Incidence of bleeding at 30 days. B) Incidence of bleeding through hospital discharge.
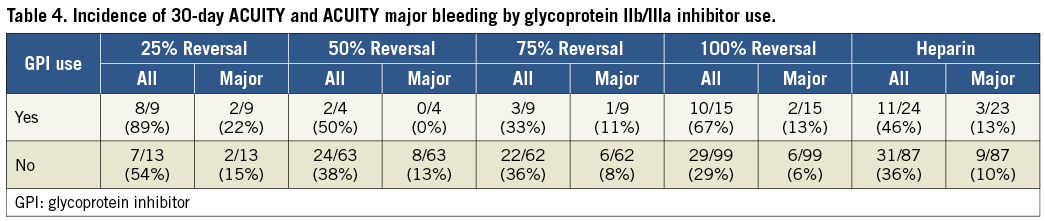
We also assessed the impact of glycoprotein IIb/IIIa inhibitor use on bleeding in both REG1-treated and heparin-treated patients. Bleeding was increased to a similar degree in all groups when glycoprotein IIb/IIIa inhibition was instituted (Table 4).
Discussion
This analysis of patients undergoing PCI in the RADAR trial demonstrates that pegnivacogin-mediated factor IXa inhibition as sole anticoagulant appears adequate for the safe conduct of PCI in an ACS population, with no evidence that partial or complete reversal of anticoagulation post procedure was associated with an increased risk of stent thrombosis.
ISCHAEMIC EVENTS
RADAR-PCI confirmed the feasibility of using pegnivacogin coupled with post-procedural reversal to suppress ischaemic events effectively during and in the early post-procedural time period, as assessed by investigator-reported thrombus on initial angiography and intraprocedural clot formation.
Approaches to the development of better anticoagulants which favourably affect both ischaemic and bleeding events include targeting novel steps in the coagulation process or use of high-potency therapeutics with short half-lives. Coagulation factor IXa is a more important determinant of thrombin generation than factor Xa or thrombin, plays a vital role in both initiation and propagation of coagulation, and is present at lower and more stable levels than other downstream targets11,12. In addition, factor IX is directly activated in response to contact with foreign surfaces, thus inhibition of factor IXa may be particularly attractive to suppress catheter-related ischaemic complications.
RADAR-PCI suggests that these considerations may translate into clinical utility. While the current study is too small to make definitive conclusions, it is reassuring that catheter-related complications noted with other strategies, notably factor Xa inhibition13, were not observed in this study. Reassuringly, given the subjective nature of thrombus identification on angiography and the open-label randomisation between REG1 and heparin, both local assessment of thrombus at angiography as well as centrally adjudicated objective endpoints were consistent. These observations suggest that factor IXa inhibition is an attractive potential target14 for effective procedural anticoagulation.
In addition, REG1 for the first time allows precise and immediate partial or complete post-procedural reversal of anticoagulation, perhaps mitigating the risk of high-level (>99%)14 intraprocedural factor inhibition through the use of a controlling strategy to titrate actively the level of anticoagulation to which the patient is exposed. The optimal level of immediate post-procedural anticoagulation has yet to be defined, but a higher incidence of acute stent thrombosis has been observed with other short half-lived agents15. We observed no evidence that immediate post-procedural reversal resulted in acute thrombotic events or stent thrombosis, as evidenced by the need for urgent target vessel revascularisation. While RADAR was not powered to assess adequately such rare events, it is notable that pegnivacogin reversal has not been associated with increased thrombin generation16.
BLEEDING
Analysis of bleeding demonstrates that at least 50% pegnivacogin reversal is required to allow early removal of femoral sheaths. While not powered to detect differences in bleeding between individual reversal arms, we observed a stepwise relationship between total and major bleeding and degree of reversal, demonstrating that acute post-procedural reversal of anticoagulation may impact on bleeding. Notably, this is consistent with prior observations demonstrating a clear relationship between the degree of anticoagulation and bleeding, while the relationship with ischaemic events is less clear17. This relationship and rate of bleeding mirrors that observed in the RADAR trial in REG1-treated patients, in which full-dose anticoagulation was administered prior to catheterisation in all patients. In contrast, the incidence of bleeding in heparin-treated patients increased from 31.3% (RADAR) to 37.8% (RADAR-PCI), probably due to the exposure to additional and higher doses of heparin in those subjects treated with PCI.
Comparison of events between REG1-treated and heparin-treated subjects is subject to the bias inherent in open-label comparisons. RADAR-PCI suggests that REG1-mediated near complete factor IXa inhibition coupled with >50% reversal results in no increase in bleeding. Indeed, the observed rate of major bleeding in the 100% immediate reversal arm was decreased by >50% relative to heparin.
CONCOMITANT MEDICATION USE
Patients in the REG1 arms were to be treated with glycoprotein IIb/IIIa inhibitors only if periprocedural findings warranted (bail-out indications). In contrast, glycoprotein IIb/IIIa inhibition was recommended in heparin-treated patients due to the acute nature of presentation required for entry into the study. At the time RADAR was designed, glycoprotein IIb/IIIa inhibitors were still recommended in addition to heparin for ACS/NSTEMI patients. In contrast, REG1 was designed to utilise a pegnivacogin dose which uniformly achieves near complete (>99.5%) factor IXa inhibition9,14, providing maximal anticoagulation.
In practice, the use of parenteral glycoprotein IIb/IIIa inhibitors was confined to a minority of heparin patients, mirroring current clinical practice, which reflected the decreasing use of GP IIb/IIIa inhibitors in North America after publication of ACUITY, the increasing awareness of bleeding complications associated with GP IIb/IIIa inhibitor use, the increasing use of upfront thienopyridines, and the (by comparison) low use of these agents in some countries represented in RADAR. In REG1-treated patients, where glycoprotein IIb/IIIa inhibitors were to be used only if angiographically indicated, their use was similar to that which has been reported in other studies with similar indications15,18-20. Their use in RADAR-PCI may have been affected by the unblinded randomisation between REG1 and heparin and unfamiliarity with a novel anticoagulant, with a resulting lower threshold for implementation of additional antithrombotic therapy.
As expected, 30-day bleeding appeared higher with the addition of glycoprotein IIb/IIIa inhibition. Our analysis suggests that the increase in bleeding risk was similar in heparin and REG1 patients, suggesting that the addition of glycoprotein IIb/IIIa inhibition does not pose an excessive bleeding risk when used in conjunction with pegnivacogin as compared with other anticoagulants; however, determining the safety and efficacy of glycoprotein IIb/IIIa use in conjunction with factor IXa inhibition will require further analysis in larger, more definitive studies.
Overall thienopyridine use and loading pre-procedure was similar between the REG1 and heparin groups. Interestingly, the use of the more potent thienopyridine prasugrel was more frequent in heparin-treated patients. The numbers of patients in this study are too small to reach definitive conclusions; however, these observations point to the potency of anticoagulation with pegnivacogin, which compared favourably with heparin in relation to ischaemic events despite the difference in thienopyridine selection.
COMPARISON WITH OTHER APPROACHES
Bivalirudin has generally been associated with ischaemic event rates that are numerically, but not statistically, higher than those in patients treated with heparin and glycoprotein IIb/IIIa inhibition, but with markedly lower bleeding rates20,21. Whether a strategy of high-level anticoagulation with near complete factor IXa inhibition coupled with reversal is more effective in suppressing short-term ischaemic complications while allowing early sheath removal without compromising bleeding is currently being tested in the REGULATE-PCI trial (NCT01848106). While heparin reversal with protamine post PCI has been explored, the safety and utility of this approach remain to be defined, and protamine has prothrombotic properties22. In contrast, anivamersen has no known biological activity in the absence of pegnivacogin reversal.
Limitations
RADAR-PCI is a post-randomisation subgroup analysis of the larger RADAR study and, as such, is underpowered for both bleeding and ischaemic comparisons. Nonetheless, the consistency of the findings with the RADAR study, together with confirmation of the expected relationship between treatment reversal and bleeding, is both convincing and encouraging. Although the number of ischaemic events is limited, we observed no concerns of thrombotic or ischaemic complications in this first significant experience with REG1 in PCI subjects.
The open-label randomisation between REG1 and heparin makes comparing both bleeding and subjective ischaemic endpoints, especially angiographic findings, between these strategies subject to potential bias. However, one might expect that greater sensitivity would be applied to the assessment of events in patients receiving open-label investigational therapy compared with a standard approach. It is therefore reassuring that the identification of thrombus and other angiographic complications remained low in pegnivacogin-treated patients and that these rates compared favourably with heparin. It is also reassuring that treatment characteristics (Table 2) remained well balanced between the groups. Indeed, the more frequent use of prasugrel in heparin-treated patients might be expected to lower ischaemic and thrombotic complications in this arm relative to REG1.
RADAR was designed in part to assess the dose of anivamersen required to allow immediate post-procedure sheath removal, and limited enrolment to femoral access. The applicability of these findings to a broader patient population requires further study; however, it is unlikely that ischaemic and thrombotic complications would be significantly affected by changes in access approach.
Conclusions
In this still limited experience, REG1-mediated factor IXa inhibition appears to be a safe and feasible anticoagulation strategy for suppressing ischaemic and thrombotic complications during PCI in a high-risk ACS population. When coupled with appropriate reversal, REG1 allows early femoral sheath removal without compromising bleeding. Additional studies of REG1 in larger and varied patient populations undergoing interventions in which transient high-level anticoagulation with rapid post-procedural reversal is attractive are warranted.
| Impact on daily practice REG1 is an investigational agent requiring further study. This analysis suggests that REG1 anticoagulation may be highly effective at suppressing ischaemic events during PCI while also favourably impacting on bleeding complications. The unique properties of the REG1 anticoagulation system may make it ideal for use in a variety of invasive procedures including other percutaneous and surgical interventions that require high-level anticoagulation and where reversal post-procedure may be particularly attractive. The current analysis lays the foundation for the REGULATE-PCI trial, which is defining the effectiveness of REG1 anticoagulation during PCI. |
Acknowledgements
We wish to acknowledge all the investigators and research staff, as well as the patients who contributed to RADAR. In addition, we wish to thank E. Cook for her contributions to the editing of the manuscript.
Funding
Regado Biosciences, Inc., supported the RADAR trial.
Conflict of interest statement
T. Povsic, L. Aberle and J. Alexander are employees of DCRI, which was provided with research support by Regado. S. Zelenkofske is an employee of Regado. R. Mehran had received modest support from Regado, AstraZeneca, Abbott, and Ortho-McNeil for advisory board membership/consulting, and research grants from Sanofi/BMS and TMC. R. Becker is an employee of DCRI, which was provided with research support by Regado, and has received modest support for advisory board membership from Regado. The other authors have no conflicts of interest to declare.

