Abstract
Aims: Thirty-day results of the double-blind, randomised Intracoronary Stenting and Antithrombotic Regimen –Rapid Early Action for Coronary Treatment (ISAR-REACT) 4 trial showed no difference in ischaemic complications and a reduction in bleeding by bivalirudin versus abciximab and heparin in 1,721 patients with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing percutaneous coronary intervention (PCI). A longer follow-up may be required to assess the whole potential benefit of a periprocedural antithrombotic therapy.
Methods and results: The primary outcome for this analysis was the composite of death, myocardial infarction or target vessel revascularisation one year after randomisation. Secondary outcome was the composite of death or myocardial infarction. At one year, the primary outcome occurred in 21.3% of patients assigned to abciximab and heparin versus 21.5% assigned to bivalirudin (hazard ratio [HR] 0.99; 95% confidence interval [CI]: 0.80-1.21; p=0.94). The combined incidence of death or myocardial infarction was 15.7% in the abciximab and heparin group versus 16.0% in the bivalirudin group (HR 0.99; 95% CI: 0.78-1.26; p=0.94). The mortality rates were 4.0% and 4.7%, respectively (HR 0.85; 95% CI: 0.54-1.34; p=0.48). At one year, no significant differences in the primary outcome were observed with abciximab and heparin versus bivalirudin in any of the subgroups analysed.
Conclusions: In patients with NSTEMI undergoing PCI, abciximab with heparin and bivalirudin provide comparable outcomes at one year, although bivalirudin reduced the rate of bleeding at 30 days. Clinical trial registration information: URL www.clinicaltrials.gov; Unique identifier NCT00373451.
Introduction
During the last 10 years, the epidemiology of myocardial infarctions has been characterised by an increase in the proportion of patients presenting with non-ST-segment elevation myocardial infarction (NSTEMI) and a decrease in those presenting with STEMI1. Patients with NSTEMI have been shown to benefit from an early invasive approach with coronary angiography and revascularisation2,3. Antithrombotic therapy is essential for the safety and efficacy of percutaneous coronary intervention (PCI), especially in the prothrombotic milieu of an on-going myocardial infarction.
Thirty-day results of the ISAR-REACT 4 trial showed that abciximab and heparin do not provide net clinical benefit (i.e., do not reduce the composite of death, large myocardial infarction, urgent target vessel revascularisation or major bleeding) but increase the risk of bleeding compared to bivalirudin4. However, the 30-day period might not be long enough to assess the whole potential benefit of a certain drug. There is a large body of evidence demonstrating that periprocedural myocardial infarction and bleeding –both affected by the antithrombotic drugs– have an impact on long-term prognosis5. Moreover, a late positive impact of abciximab on the development of restenosis has also been reported6,7.We therefore performed one-year follow-up of patients enrolled in the ISAR-REACT 4 trial to assess the long-term outcome associated with the two regimens.
Methods
STUDY POPULATION
A detailed description of the ISAR-REACT 4 trial has recently been published4. In brief, this randomised, double-blind multicentre trial included 1,721 biomarker positive patients (with either troponin or creatinine kinase myocardial band (CK-MB) above the upper limit of normal), presenting with the symptoms of unstable angina and a coronary anatomy amenable to PCI.
DETAILS OF STUDY PROTOCOL
Participating centres followed an early invasive strategy for patients presenting with NSTEMI. All patients received aspirin (325-500 mg) and 600 mg of clopidogrel before randomisation. After the decision to perform PCI, patients were randomised by means of sealed opaque envelopes. Patients assigned to abciximab received a bolus of 0.25 mg/kg of abciximab, followed by an infusion of 0.125 μg/kg/min (maximum of 10 μg/min) for 12 hours, and a bolus of 70 units/kg of unfractionated heparin. Patients in the bivalirudin group received a bolus of 0.75 mg/kg of bivalirudin, followed by an infusion of 1.75 mg/kg/hr for the duration of the procedure. Most procedures were performed with access through the femoral artery, although access through the radial artery was permitted. Post-procedural antithrombotic therapy included aspirin (80-325 mg) indefinitely and clopidogrel for at least six months.
FOLLOW-UP, ENDPOINTS AND STUDY DEFINITIONS
All patients were either seen by their physician or interviewed by phone at 30 days and one year. Those with cardiac complaints underwent a complete clinical, electrocardiographic, and laboratory evaluation. If patients suffered an adverse event at another hospital, appropriate source documents were solicited (including discharge summaries, laboratory values and angiograms). Family doctors, referring cardiologists, patients or their relatives were contacted for additional information if required.
The primary outcome of the current analysis was the combined incidence of death from any cause, myocardial infarction, or target vessel revascularisation at one year after randomisation (major adverse cardiac events, MACE). Vital status was obtained from hospital records, death certificates or phone contact with relatives of patients or their attending physicians. The diagnosis of myocardial infarction required the presence of new pathologic Q-waves (≥30 msec in duration and ≥0.1 mV in depth) in ≥2 contiguous or adjacent electrocardiographic leads, or elevation of CK-MB isoenzyme (or total CK if CK-MB was not available) ≥3 times the upper limit of normal. Target vessel revascularisation (TVR) was defined as any ischaemia-driven repeat PCI or bypass surgery of the target vessel. Stent thrombosis was considered to have occurred when the criteria for definite stent thrombosis of the Academic Research Consortium were met8. All events were adjudicated and classified by an events adjudication committee blinded to the treatment assignments.
STATISTICAL ANALYSIS
Data are presented as counts (followed by percentages in parentheses), medians [with interquartile range in parentheses] or Kaplan-Meier estimates (%). We calculated the hazard ratios (HR) with 95% confidence intervals (CIs) associated with therapy with abciximab and unfractionated heparin versus bivalirudin by the use of Cox proportional hazard models. The proportional hazards assumption was checked by the method of Grambsch and Therneau9 and was fulfilled in all cases in which we used Cox proportional hazard models.
Data for patients lost to follow-up who did not have the event of interest were censored at the date of the last follow-up.
Prespecified subgroup analysis involved subgroups dichotomised by median age (68.3 years), sex, presence of diabetes, median body mass index (27.3 kg/m2), median glomerular filtration rate (83 ml/min) and median troponin level on admission (0.12 µg/L).
Heterogeneity in treatment outcomes across subgroups was examined by assessing the interaction between the assigned treatment and the baseline variable defining the subgroup with respect to the primary outcome. This was done by entering the interaction term into the respective Cox proportional model.
All analyses were performed using S-PLUS statistical package (Version 4.5; Insightful Corporation, Seattle, WA, USA). A two-sided p-value of less than 0.05 was considered to indicate statistical significance.
Results
The baseline characteristics of the 1,721 patients enrolled in the randomised ISAR-REACT 4 trial have previously been reported4. At one year, follow-up was complete in all but 29 patients (1.7%; 17 patients in the abciximab group and 12 patients in the bivalirudin group).
CLINICAL OUTCOMES AT ONE YEAR
Table 1 summarises the one-year clinical outcomes in both groups. The incidence of the primary outcome - the composite of death, myocardial infarction or target vessel revascularisation - was 21.3% (n=182) in the abciximab plus heparin group and 21.5% (n=184) in the bivalirudin group (HR 0.99, 95% CI: 0.80–1.21, p=0.94; Figure 1).
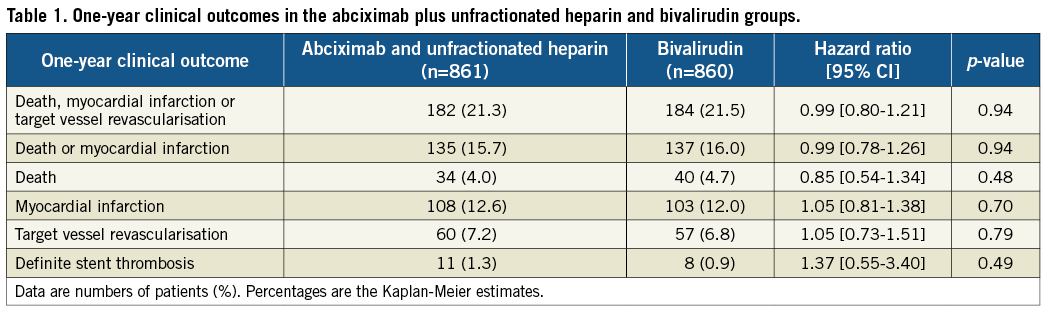
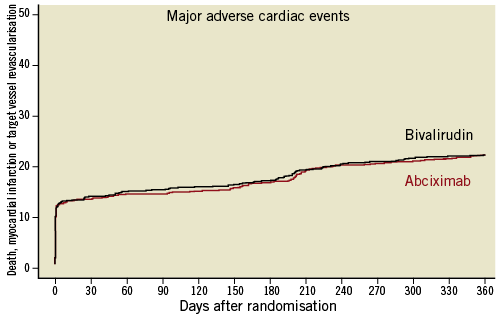
Figure 1. Kaplan-Meier curves of the composite of death, myocardial infarction or target vessel revascularisation.
The secondary endpoint, the composite of death or myocardial infarction, occurred in 15.7% (135 patients) in the abciximab group and 16% (137 patients) in the bivalirudin group (HR 0.99, 95% CI: 0.78–1.26, p=0.94; Figure 2).
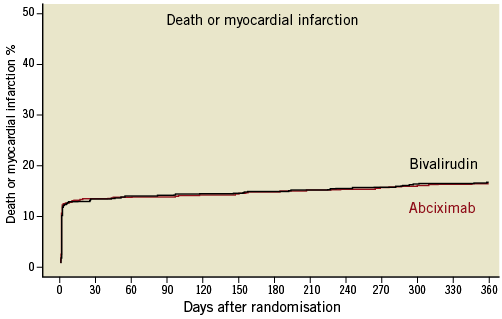
Figure 2. Kaplan-Meier curves of the cumulative incidence of death or myocardial infarction.
At one year, 34 patients (4%) in the abciximab group and 40 patients (4.7%) in the bivalirudin group had died (HR 0.85, 95% CI: 0.54–1.34, p=0.48).
The cumulative incidence of myocardial infarction at one year was 12.6% (108 patients) in the abciximab group and 12% (103 patients) in the bivalirudin group, respectively (HR 1.05, 95% CI: 0.81–1.38, p=0.70).
Revascularisation of the target vessel was performed in 60 patients (7.2%) in the abciximab group versus 57 patients (6.8%) in the bivalirudin group (HR 1.05, 95% CI: 0.73–1.51, p=0.79).
Definite stent thrombosis occurred in 1.3% (11 patients) and 0.9% (eight patients), respectively (HR 1.37, 95% CI: 0.55–3.40, p=0.49).
Analysis of the primary outcome - the composite of death, myocardial infarction or target vessel revascularisation - in the prespecified subgroups defined by age, sex, diabetes, body mass index, renal function and troponin level revealed no significant interaction between the subgroups and the treatment effects of the study groups (Figure 3).
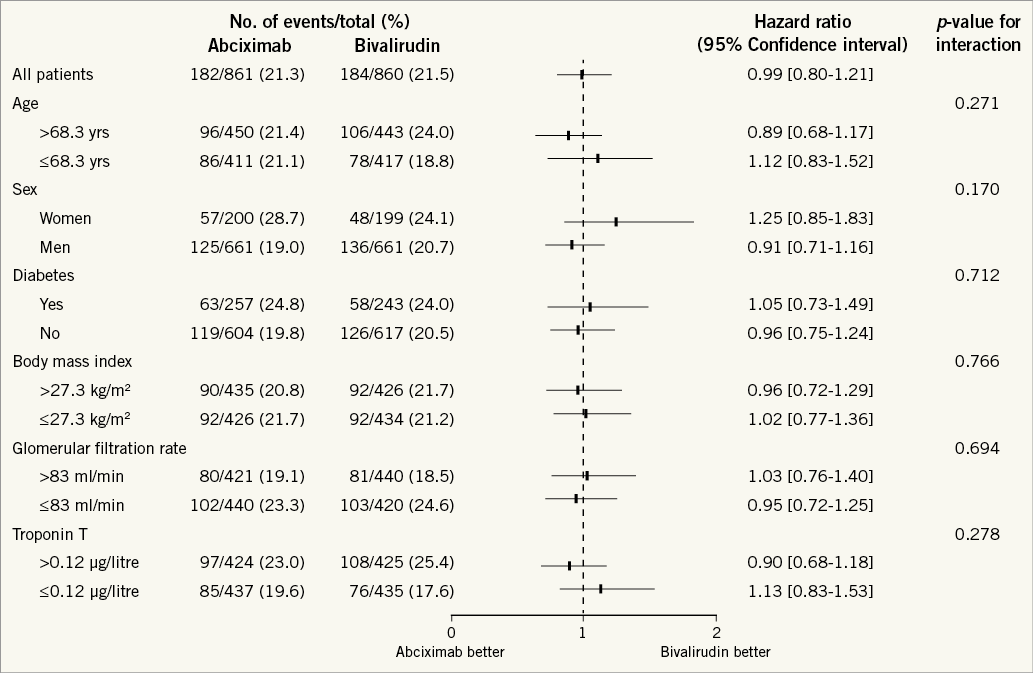
Figure 3. One-year risk of the composite of death, myocardial infarction or target vessel revascularisation related to assignment to abciximab and heparin or bivalirudin in prespecified subgroups. Cut-off values for age, body mass index, glomerular filtration rate and troponin T are their medians in the study population.
No significant interaction was found between reduced TIMI flow at baseline and treatment effect (pinteraction = 0.61). More specifically, at one year, patients with TIMI flow grade <3 showed a MACE rate of 27.6% (111/404) in the abciximab group and 26.1% (111/426) in the bivalirudin group (HR 1.07, 95% CI: 0.83–1.40) and patients with TIMI flow grade 3 showed a MACE rate of 15.4% (70/455) in the abciximab group and 16.8% (72/432) in the bivalirudin group (HR 0.92, 95% CI: 0.66–1.27).
Discussion
This updated follow-up of patients enrolled in the randomised ISAR-REACT 4 trial shows that, as within 30 days, adjunct use of abciximab plus heparin continued to show no benefit in reducing ischaemic complications up to one year after the index procedure. Furthermore, the observed advantage of bivalirudin in terms of reduction in bleeding events within 30 days was not translated into a reduced one-year mortality rate.
Patients presenting with elevated biomarkers, i.e., patients with NSTEMI, differ substantially from those presenting with normal biomarkers. Therapeutic implications of the separation of these two entities have, amongst others, been illustrated by the randomised ISAR-REACT 2 trial, which revealed that the addition of the glycoprotein IIb/IIIa inhibitor abciximab to heparin is associated with a reduction in ischaemic endpoints only in troponin-positive patients with NSTE-ACS10. Of note, the reduction in ischaemic endpoints with abciximab was not associated with a meaningful increase in bleeding in that study. Analysis of the one-year results revealed persistent benefit from abciximab in that study11. The current ISAR-REACT 4 trial was specifically designed to investigate whether the combination of abciximab and heparin is also superior to the direct thrombin inhibitor bivalirudin in patients with NSTEMI undergoing invasive treatment with PCI. After one year of follow-up, however, abciximab and heparin continued to show no benefit over bivalirudin. Instead, there was a significant increase in protocol-defined major bleeding and TIMI minor bleeding with abciximab at 30 days4. Notably, even TIMI minor bleeding has been shown to be associated with late mortality5. On the other hand, late clinical benefit has been suggested with abciximab by reducing restenosis via inhibition of the vitronectin (αVß3) receptor7. Since there was only a trend of reduced TLR rates with abciximab in the predecessor ISAR-REACT 2 trial (13.6% vs. 16.8%; p=0.08)7, it is perhaps not surprising that there was no significant reduction in restenosis in ISAR-REACT 4, which had a much higher penetration of drug-eluting stents (>88% vs. 49%).
A reduction in bleeding with bivalirudin has been a consistent finding of contemporary trials in which it was compared against a glycoprotein IIb/IIIa inhibitor and heparin12,13 and also against heparin monotherapy14. The prognostic implications of periprocedural complications, including bleeding, have been the focus of extensive research. It could be shown that bleeding is a strong and independent predictor of long-term outcome after PCI5.
Therefore, why the reduction in bleeding with bivalirudin did not translate into longer-term benefit in NSTEMI patients enrolled in ISAR-REACT 4 is not clear. One reason might be that the trial was largely underpowered for rare events such as mortality. Another potential reason is the different impact of bleeding on mortality according to clinical presentation.
Although patients with NSTE-ACS have a lower in-hospital mortality than STEMI patients, this difference in mortality diminishes over time and is even reversed during longer-term follow-up in some studies15,16. Therefore, current guidelines stress longer-term outcomes when recommending treatment strategies in patients suffering NSTE-ACS17. Recent analyses suggest that patients with NSTEMI are exactly the group of patients in whom a reduction in bleeding might translate into a reduction in mortality18.
The results of the current analysis are concordant with those reported from the larger ACUITY trial which enrolled patients with NSTE-ACS19. In that study, there was also no difference in ischaemic events or mortality after one year of follow-up in the overall cohort19 or in those undergoing PCI20.
However, in patients presenting with STEMI, bivalirudin has not only been shown to reduce bleeding but also to reduce short and longer-term overall mortality, as well as cardiac mortality, compared to the combination of glycoprotein IIb/IIIa inhibitors and heparin21. Although there was a small increase in acute (<24 hours) stent thrombosis with bivalirudin in STEMI patients in the Harmonising Outcomes with Revascularisation and Stents in Acute Myocardial Infarction (HORIZONS AMI) trial13, the incidence of very late stent thrombosis was actually lower in bivalirudin-treated patients, suggesting a possible interaction between a reduction in bleeding and later ischaemic events21. Several different mechanisms of how bleeding affects mortality and ischaemic events have been suggested and these are discussed elsewhere22.
A different pattern in bleeding complications has been observed in STEMI and NSTEMI patients. While the incidence of TIMI major bleeding was higher than the incidence of TIMI minor bleeding in STEMI patients enrolled in the HORIZONS AMI trial13, NSTEMI patients enrolled in ISAR-REACT 44 and ACUITY12 suffered from TIMI minor bleeding more than twice as often as from TIMI major bleeding in all treatment groups. This may indicate different bleeding risks across the spectrum of ACS patients, and should be the subject of further investigation.
Several analyses of biomarker negative patients23, as well as of patients with NSTEMI24 and STEMI25, have suggested a lesser ability of bivalirudin to prevent ischaemic events than abciximab and heparin. However, the current and other longer-term analyses provide reassurance that this does not translate into adverse longer-term clinical outcome with bivalirudin.
The current analysis bears several limitations. The most important limitation is the fact that the ISAR-REACT 4 trial was powered for the primary, 30-day endpoint and certainly lacks statistical power for detecting difference in rarer events, such as death.
As with every trial, the results should only be interpreted within the special circumstances in which it was performed. Since newer and more potent antiplatelets than clopidogrel (which was used in this trial), such as prasugrel26 and ticagrelor27, are now available, the results of bivalirudin vs. abciximab and heparin among patients receiving such agents are not known.
We recommended a minimum duration of dual antiplatelet therapy of six months. Current guidelines recommend a longer duration of therapy28,29. Because individual data on the duration of dual antiplatelet therapy were not collected we are unable to assess the impact of this factor on one-year outcome.
In summary, this one-year analysis reveals that in patients with NSTEMI undergoing PCI abciximab with heparin and bivalirudin provide comparable clinical outcomes including mortality, although bivalirudin caused less bleeding within 30 days.
Funding
The ISAR-REACT 4 trial was supported in part by Nycomed Pharma GmbH, Unterschleißheim, Germany (former distributor of bivalirudin in Europe), and the grant KKF 04-06 [974404] from Deutsches Herzzentrum, Munich, Germany.
Conflict of interest statement
P. Berger has served as a consultant for Medicure, Janssen and BMS/Sanofi and has received grants/grants pending from Helena, Novartis, Tethys, Thrombovision, Accumetrics, Astra Zeneca, Haemoscope, The Medicines Company, BMS/Sanofi and Lilly/DS. A. Kastrati has received payment for lectures including service on speakers bureaus from Astra Zeneca, Bristol Myers Squibb, Lilly, The Medicines Company, Daiichi Sankyo and MSD. J. Mehilli has received payment for lectures including service on speakers bureaus from Lilly/Daiichi Sankyo, Terumo and Abbott Vascular. F.-J. Neumann has received grants/grants pending from The Medicines Company and Eli Lilly, Novartis, Astra Zeneca, Roche, Boston Scientific, Edwards, Medtronic and Biotronic, and support for travel to meetings for the study or for other purposes from The Medicines Company and Eli Lilly, has served as a consultant for Astra Zeneca and Eli Lilly, and has received payment for lectures including service on speakers bureaus from Astra Zeneca, Daichi Sankyo, Medtronic, Biotronic, Edwards and Siemens. H. Schühlen has served as a consultant for Lilly, Daiichi-Sankyo and Bayer and has received payment for lectures including service on speakers bureaus from Lilly, Daiichi Sankyo, Astra Zeneca, Iroko Cardio and Bayer. The other authors have no conflicts of interest to declare.

