Anticoagulation is a crucial component of the pharmacological cocktail administered to patients with non-ST-elevation acute coronary syndromes (NSTE-ACS). The available options include unfractionated heparin (UFH), enoxaparin, bivalirudin and fondaparinux. In NSTE-ACS patients undergoing percutaneous coronary intervention (PCI), a general recommendation is to continue the initial therapy and avoid switching between antithrombins, with the exception of adding UFH to fondaparinux1. The scope of anticoagulation during PCI is to suppress thrombotic events during and in the early term after the procedure. However, fine-tuning the balance between ischaemic and bleeding complications is a challenge with current anticoagulant pharmacotherapy. Bleeding per se may significantly jeopardise the clinical outcomes2, hence careful selection and dosing of anticoagulants is needed, particularly in the era of antiplatelet agents more potent than clopidogrel.
The ideal anticoagulant reduces thrombus formation with minimal bleeding, and is easily administered and monitored. An additional benefit of such an ideal compound would include the possibility to administer a reversal agent in case of clinical need (e.g., bleeding), or when anticoagulation is no longer necessary (e.g., for facilitating early sheath removal after catheterisation). Marketed anticoagulants’ lack of one or more of these ideal features (Table 1) has prompted investigation into alternative agents. Otamixaban - an intravenous direct factor Xa inhibitor –recently failed to hit the “sweet spot” of reducing both ischaemic and bleeding complications in a large trial3.
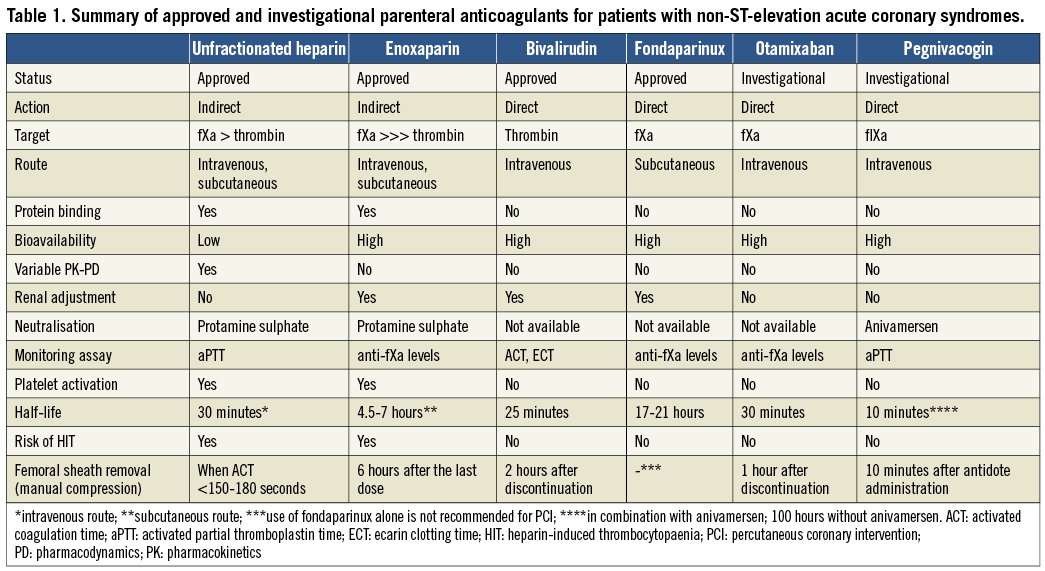
While currently used anticoagulants typically target factors IIa (bivalirudin, UFH, enoxaparin) and Xa (UFH, enoxaparin, fondaparinux) of the coagulation cascade, a novel approach aimed at suppressing the activity of factor IXa, an upstream protease activated in the early steps of coagulation, is attractive for a number of reasons. First, factor IXa plays a central role in the generation of factor Xa and in the initiation and propagation of clot on the surface of activated platelets. Second, factor IXa is responsible for the activation of factor Xa through the intrinsic pathway and is directly activated in response to contact with foreign surfaces, which may become useful in the setting of catheter-based interventions. Third, factor IXa is present at lower and more stable concentrations than other downstream proteases, which makes its inhibition more consistent and predictable. Fourth, inhibition of factor IXa results in prolonged activated partial thromboplastin time, similar to its pathophysiological correlate haemophilia B, thus allowing easy monitoring of anticoagulation with a simple blood test.
In this issue of EuroIntervention, Povsic and colleagues report on the results of PCI patients included in the RADAR trial, a phase II investigation of a factor IXa inhibitor named pegnivacogin (formerly known as RB006), used for anticoagulation in patients with NSTE-ACS4,5. Pegnivacogin is a single-strand RNA which belongs to the class of aptamers, modified oligonucleotides that bind to proteins based on their three-dimensional configuration. Unique to aptamers is the possibility to engineer their own complementary reversal agent based on Watson-Crick base pairing. The controlling agent of pegnivacogin, named anivamersen (formerly known as RB007), has no biological effect other than rapidly and irreversibly binding to pegnivacogin, resulting in its dissociation from the factor IXa binding site and prompt reversal of the anticoagulant effect. In the RADAR trial, at least 50% reversal with anivamersen was required to allow safe sheath removal with manual compression or vascular closure devices immediately after cardiac catheterisation via the femoral approach. Interestingly, in control patients treated with heparin, the sheath was removed from coronary angiography or PCI at a median of 184 minutes (versus 23 minutes in the pegnivacogin/anivamersen group). The RADAR-PCI substudy extends those prior observations with encouraging angiographic findings and ischaemic endpoints in support of further clinical testing of pegnivacogin for the safe conduct of PCI in NSTE-ACS4. Indeed, among 388 patients undergoing PCI of the 640 enrolled in RADAR, catheter-related complications (e.g., thrombus formation during PCI) did not significantly exceed those of the heparin controls. Also, reassuringly, the composite of death, myocardial infarction, urgent revascularisation and recurrent ischaemia was statistically similar (and numerically lower) with pegnivacogin compared with heparin (4.4% vs. 7.3%, p=0.30), despite prasugrel being used more frequently in heparin patients, probably due to unfamiliarity with the investigational anticoagulant. A minority of patients received glycoprotein IIb/IIIa inhibitors (GPIs) during PCI in both the pegnivacogin and heparin groups, despite GPIs being recommended by protocol in the heparin arm. Not surprisingly, thirty-day bleeding was increased with the addition of GPIs, but there were no notable differences between patients receiving pegnivacogin and those receiving heparin.
Although representing a non-randomised subgroup analysis and being largely underpowered for clinical endpoints, the findings from RADAR-PCI are reassuringly consistent with those from RADAR, and represent a positive step in view of the further clinical development of pegnivacogin. However, they also share with RADAR some concerns that should be underscored. First, the administration of pegnivacogin and heparin was open-label, raising the potential for ascertainment bias, particularly when subjective endpoints are considered. Second, the RADAR trial was halted prudentially after three patients had allergic-like reactions shortly after pegnivacogin administration, an issue which warrants consideration. It cannot be excluded, however, that those cases represent just a random clustering of otherwise rare events in subjects with a prior history of allergic reactions. Third, whether prompt reversal of anticoagulation after PCI results in withdrawal of protection with potential for increased ischaemic events needs further investigation in larger populations. Finally, the relative role of pegnivacogin in patients undergoing PCI with other approaches (e.g., radial), clinical presentations (e.g., ST-elevation myocardial infarction), different controls (e.g., UFH plus systematic protamine; bivalirudin) or higher proportions of newer antiplatelet agents (e.g., prasugrel, ticagrelor) remains unexplored.
Intriguingly, the theoretical benefit of early and safe sheath removal may apply to other catheter-based interventions (e.g., transcatheter aortic valve replacement), which makes further investigation of aptamers attractive, although this hypothesis needs rigorous verification6. The history of antithrombotic drug development contains many agents which failed to confirm the positive results of early phase II investigations when larger studies were conducted on a more clinical basis. The REGULATE-PCI trial (NCT01848106) is designed to investigate the efficacy and safety of the pegnivacogin/anivamersen combination compared to bivalirudin in 13,200 patients undergoing PCI, however enrollment has been paused at the time of writing this editorial. The Data Safety and Monitoring Board (DSMB) is conducting an unplanned full risk-benefit analysis of the more than 3,200 patients enrolled to date, with a focus on serious adverse events related to allergic reactions. Subsequently the United States Food and Drug Administration (FDA) has placed a “clinical hold” on the study. Recommendations to re-initiate enrollment and dosing in the trial will be reliant on FDA agreement. Are aptamers the next big thing in the anticoagulation field? Time will tell.
Conflict of interest statement
D. Capodanno is a member of the advisory board of, and has received speaker’s honoraria from Eli Lilly, Daiichi Sankyo and AstraZeneca.

