Abstract
Aims: To evaluate the efficacy and safety of intravenous enoxaparin as an alternative to unfractionated heparin (UFH) as antithrombotic therapy in unselected patients undergoing percutaneous coronary intervention (PCI).
Methods and results: Eight hundred and seventy-six (876) consecutive eligible patients undergoing PCI were prospectively randomised to either intravenous enoxaparin 0.75 mg/kg or dose-adjusted UFH in this open-label study that was prematurely stopped due to slow recruitment. Randomisation was stratified on elective PCI or PCI for acute coronary syndrome (ACS). The primary endpoint was a combination of death, myocardial infarction, unplanned target vessel revascularisation and major bleeding at 30 days. Secondary endpoint was a composite of major and minor bleeding and thrombocytopenia < 50x109.
The primary endpoint of intravenous enoxaparin did not differ from those of UFH (5.5% vs. 7.0%, p=ns) whereas safety endpoints were reduced with enoxaparin compared to UFH (9.9% vs. 20.0%, p<0.001). Among 229 (26%) patients presenting with ACS, the incidence of both, the primary and secondary endpoints, was lower with enoxaparin as compared to UFH (1.8% vs. 12.9% and 14.2% vs. 31%, p<0.001 and p=0.003, respectively).
Conclusions: Due to the premature halting of the study and the low event rate, these data are observational only, and no definite conclusion could be made concerning efficacy and safety of intravenous enoxaparin as an alternative to UFH in unselected patients undergoing PCI.
Introduction
The optimal antithrombotic therapy during percutaneous coronary interventions (PCI) is still controversial. An ideal drug should have predictable pharmacokinetics and should be equally effective in stable patients and those presenting with acute coronary syndrome (ACS). Bleeding complications should be minimised as major and even minor haemorrhages and the use of transfusions affect short- and long-term prognosis adversely1-3. Adverse interactions with complex additional anti-ischaemic and antiplatelet regimens should be minimal.
While unfractionated heparin (UFH) is currently recommended as the standard of care for patients undergoing elective PCI, current guidelines for ACS patients have been updated, including alternatives to UFH such as bivalirudin and enoxaparin4-6. For both subsets of patients UFH has limitations, including a narrow therapeutic window, unpredictable pharmacokinetics, platelet activation and the inability to inhibit clot-bound thrombin. Substantial variability of the dose requirements to prevent thrombotic complications in order to avoid bleeding complications make monitoring during the procedure necessary, often resulting in frequent dose adjustments7. This variability is mainly due to differences in concentrations of heparin-neutralising plasma proteins and rates of heparin clearance. Thus, for optimising the balance between prevention of clot formation and bleeding complications, dose adjustments according to activated clotting time (ACT) are commonly used.
Low molecular weight heparins (LMWH) may be an attractive alternative. In contrast to UFH their pharmacokinetics are highly predictable and they can be used without anticoagulation monitoring. In patients with acute coronary syndromes enoxaparin has been shown to be superior to UFH for prevention of ischaemic events8-10. The safety and efficacy of LMWH, mostly enoxaparin, in patients undergoing PCI have been evaluated in several studies11-14. The rates of bleeding or ischaemic complications have been found to be either lower or comparable to those with UFH11-14. In the SYNERGY trial and the phase A of the A-Z trial patients with acute coronary syndromes were randomised to UFH or subcutaneously administered enoxaparin15,16. Pre- and post-randomisation crossover between the two substances was substantial, however, and may have affected rates of bleeding complications. In the subgroups of patients undergoing PCI a similar rate of major thrombotic complications could be demonstrated for both regimens15,17. Based on these findings, current guidelines consider LMWH a reasonable alternative to UFH in patients with unstable angina and non-ST-elevation myocardial infarction undergoing PCI (class IIa, level of evidence B) and in patients with ST-elevation myocardial infarction (STEMI) already pre-treated with enoxaparin (class I, level of evidence B)4-6.
The aim of the present study was to demonstrate the non-inferiority of the routine use of 0.75 mg/kg enoxaparin given intravenously versus optimised UFH in a mixed, real world population of stable and ACS patients undergoing PCI, after exclusion of patients receiving antithrombin therapy prior to arrival in the cathlab.
Methods
Patients
All patients presenting to our tertiary care hospital as candidates for PCI with stable coronary artery disease or ACS (unstable angina, non-ST-elevation myocardial infarction, ST-elevation myocardial infarction) were considered eligible for randomisation in the absence of the following exclusion criteria: upstream administration of antithrombotic or thrombolytic therapy prior to presentation in the cathlab (all ACS patients transferred from other hospitals or pre-treated in the pre-hospital period), cardiogenic shock, active bleeding, known intolerance to UFH or LMWH, pregnancy, lack of informed consent.
The study was conducted according to the Declaration of Helsinki and local regulations. Approval for the trial was obtained from both the institutional and the regional ethics committee. All patients in the study gave written informed consent.
Design
The study design was an open label, 1:1 randomised, controlled study comparing intravenous enoxaparin with intravenous UFH in patients undergoing PCI. Randomisation was blinded using computer-generated, random numbers in sealed envelopes and an allocation scheme providing stratification for stable patients with elective indications for PCI, and ACS patients with urgent indications for PCI, respectively.
Patients were randomised to either enoxaparin (0.75 mg/kg IV) without further adjustments during PCI, or weight-adjusted UFH (60 U/kg IV) as an initial bolus, with further adjustment to achieve a goal ACT of 300 seconds, or 250 seconds for patients receiving glycoprotein IIb/IIIa inhibitors. UFH was adjusted according to repetitive ACT measurements. IfACT was below 250 seconds or 200 seconds respectively (for patients receiving glycoprotein IIb/IIIa inhibitors), an additional bolus of 2500 E was given and ACT measurement was repeated. All patients not already on chronic aspirin therapy received 500 mg aspirin IV before PCI. A loading dose of clopidogrel (300 – 600 mg) was administered orally immediately with PCI, followed by 75 mg daily.
All procedures were performed via femoral access. The interventional technique and concomitant medical treatment was at the discretion of the treating staff cardiologists, all of whom were experienced for elective and emergency interventions. Management followed published guidelines4.
Sheath removal in patients with UFH treatment was permitted after a documented ACT value <200 sec whereas in enoxaparin-treated patients sheath removal was recommended as early as conveniently possible. Vascular access closure devices were used at the discretion of the operator and followed local guidelines.
Systematic follow-up was performed at 30 days and consisted of a telephone interview with the patient and his treating physician. Adverse events after hospital discharge were documented by patient visits and chart reviews.
Endpoints
All endpoints were assessed without knowledge of the treatment allocation by our quality and complication assessment service which included visits from a specially trained quality control nurse to all patients as well as review of the charts and laboratory results.
The primary endpoint was a composite of all cause-death, myocardial infarction (MI), unplanned target vessel revascularisation (PCI or CABG) and major bleeding at 30 days. MI was defined in elective PCI patients as > 2-fold increase of creatine kinase (CK) above the upper limits of normal with concomitant elevation of troponin I, and in patients with ACS as a renewed rise of CK > 50%. Major bleeding was defined as intracranial, intraocular or retroperitoneal bleeding confirmed by imaging, a fall of haemoglobin levels >3 g/dl, or haemorrhages requiring transfusion of at least two units of packed red blood cells (PRBC).
The secondary endpoint was a combined safety endpoint including all major and minor bleeding and thrombocytopenia < 50x109/L at 24 hours. Minor bleeding was defined as all clinically meaningful haemorrhages, such as gastro-intestinal bleeding or gross haematuria, which did not fulfil the criteria for major bleeding. Furthermore, a deep haematoma at the puncture site was considered as minor bleeding if its diameter surpassed 5 cm. Sonography of the puncture site was performed routinely if a puncture site complication was suspected.
Statistical analysis
The study was planned as a non-inferiority study using the null-hypothesis π (enoxaparin) ≥ π (UFH) + δ, where π are the event rates and the non-inferiority margin δ was set to 3% based on previous study results18,19. For an event rate of death, myocardial infarction, unplanned target vessel revascularisation and major bleeding at day 30 of 10%, a two-sided significance level of 5% and a power of 80%, a sample size of 1,220 subjects for each group was calculated.
Data storage and analysis was performed by an independent data centre (la volta statistics, Zurich, Switzerland) using the SPSS statistics package. Continuous variables are presented as means with standard deviations and discrete variables are presented as frequencies and percentages. Student’s t test was used for comparison of continuous variables, and Fisher’s Exact Test and Pearson Chi Square with continuity correction for categorical variables. A predefined analysis addressed the subgroups of patients with elective indications for PCI and patients with ACS.
A planned interim analysis after inclusion of 800 patients performed by an independent and blinded data monitoring committee revealed a lower than expected incidence of the primary endpoint as well as a slow recruitment rate due to the exclusion of patients pretreated with antithrombin agents. The study was therefore halted prematurely, since it seemed unrealistic to meet the precalculated sample size of patients to achieve a power of 80%.
Results
Overall 876 patients qualified for the study and were randomised. Patient enrolment and flow of study participants are shown in Figure 1.
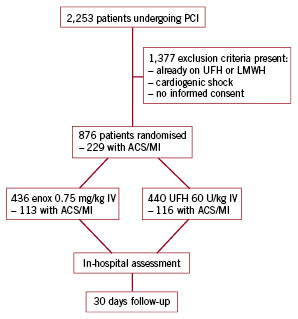
Figure 1. Diagram showing enrolment, flow and follow-up of study participants. UFH: unfractionated heparin; LMWH: low molecular weight heparin; enox: enoxaparin; ACS: acute coronary syndrome; MI: myocardial infarction; PCI: percutaneous coronary intervention
Baseline patient characteristics are shown in Table 1.
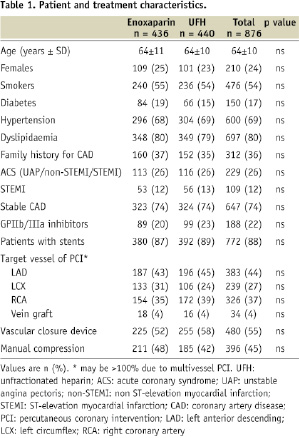
Both patient groups were well matched in regard to age, sex, presence of cardiovascular risk factors, clinical presentation, target vessel of PCI, use of stents, glycoprotein IIb/IIIa inhibitors and vascular closure devices. Notably, 772 (88%) patients were treated with stents, 78% of these being drug-eluting stents. Glycoprotein IIb/IIIa inhibitors were administered in 188 (22%) interventions, and puncture site closure devices were used in 480 (55%) patients.
Table 2 shows the frequency of the primary and secondary endpoints.
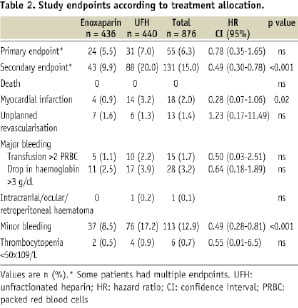
The combined primary endpoint occurred in 55 (6.3%) patients, 5.5% in the enoxaparin group and 7% in the group with UFH (p < 0.003 for non inferiority of enoxaparin vs. UFH).
The secondary endpoint occurred in 43 (9.9%) patients treated with enoxaparin, compared to 88 (20%) patients in the UFH group (p < 0.001). This statistically significant difference however was mainly driven by a lower incidence in minor bleeding (8.5% for enoxaparin vs. 17.2% for UFH, p < 0.001). Profound thrombocytopenia was observed in two patients with enoxaparin and four with UFH (p = ns). Of note, there was no difference in the occurrence of bleeding between manual compression and the closure device group.
Other important and potentially treatment-related events are also listed in Table 3.
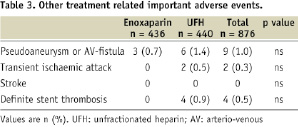
All cases of subacute stent thrombosis occurred in patients randomised to UFH during the initial procedure. Unplanned rehospitalisation occurred in 2.8% of enoxaparin-treated patients and in 4.5% of patients treated with UFH (p = ns).
Among 229 (26%) patients with ACS and primary PCI, the incidence of the primary endpoint was reduced in patients with enoxaparin in comparison to UFH (1.8% vs. 12.9%, p< 0.001). Treatment with enoxaparin vs. UFH also lowered the rate of the secondary endpoint (14.2% vs. 31.0%, p = 0.03). This statistically significant difference was driven by an increase in occurrence of major bleeding complications and myocardial infarction (Figure 2).
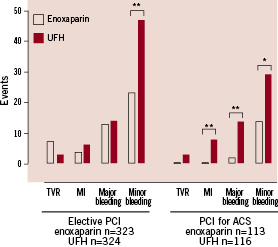
Figure 2. Incidences of endpoints in predefined patient groups with elective indications for PCI and with an acute coronary syndrome (ACS) as indication for percutaneous coronary intervention (PCI), respectively. ** p <0.01, * p <0.05 for differences between enoxaparin vs. UFH. TVR: target vessel revascularisation; MI: myocardial infarction; UFH: unfractionated heparin
Discussion
The aim of this trial was to compare the routine use of 0.75 mg/kg enoxaparin IV versus standard treatment with UFH in patients undergoing PCI for stable and unstable coronary artery disease not yet pretreated with an antithrombin agents. Although enoxaparin treatment showed the same primary event rate as UFH and resulted in fewer bleeding complications in our study, definite conclusions could not be made, since the study was underpowered due to early termination and low event rate.
For interpretation and comparison with prior trials several characteristics of trial design should be taken into account. No prolonged antithrombin treatment after PCI was given in our patients. We used the intravenous administration of both enoxaparin and UFH immediately before PCI, whereas in most trials comparing enoxaparin with UFH, enoxaparin was given subcutaneously, thus allowing PCI to be performed between one and eight hours after application20. This intravenous administration regimen is more appropriate for routine use in a busy interventional setting and for immediate treatment of patients with an acute myocardial infarction.
The STEEPLE trial, a randomised controlled study, compared two dosing regimens of intravenous enoxaparin (0.5 mg/kg and 0.75 mg/kg) and UFH in patients undergoing elective PCI11. The incidence of major bleeding was significantly reduced in both enoxaparin groups as compared to the UFH group. No significant difference among the three groups was observed with regard to the composite endpoint of death, myocardial infarction or urgent target revascularisation within 30 days. Of note, 92% of patients in the 0.75 mg/kg group reached target anticoagulation levels, defined as an anti-Xa value between 0.5-1.8 IU/ml. These results lend support for the dose used in our present study.
A further important feature of our study was the avoidance of pre- and post-randomisation crossover between enoxaparin and UFH, which occurred in a substantial part of the patients in the SYNERGY trial and which has been shown to increase bleeding complications15. Bearing these aspects in mind our results are fully compatible with the recent results in the more stringently selected patient subsets of the STEEPLE trial11.
The efficacy and safety of 0.75 mg/kg enoxaparin IV for antithrombotic treatment in all groups of PCI patients as shown in our study may be due to several factors. First, enoxaparin has a predictable pharmacokinetic profile leading to therapeutic anti-Xa levels in almost all patients without need of monitoring11. Second, platelets are less activated by enoxaparin. Finally, thrombocytopenia is rare and, where occurring in our study, may have been partly due to the concurrent use of glycoprotein IIb/IIIa inhibitors. Especially in patients undergoing PCI for unstable coronary disease, the single dose of IV enoxaparin used in this study showed a more favourable safety profile than UFH. Since glycoprotein IIb/IIIa inhibitors were almost exclusively given in patients with ACS, the higher overall rate of bleeding in this patient subset may be due to the combination of glycoprotein IIb/IIIa inhibitors and UFH or enoxaparin, respectively.
Limitations
There are several limitations of our study. The major limitation is the premature termination of the trial before reaching the precalculated sample size and the lower than expected event rate to achieve a power of 80%. Therefore, no direct conclusions can be drawn and the study may be seen as observational only.
Due to the exclusion of all patients already on UFH or enoxaparin before reaching the cathlab because of pre-hospital treatment, treatment in the emergency room, or transfer from another hospital, only 26% of recruited patients had ST-elevation and non-ST-elevation ACS. This is considerably lower than the proportion of ACS patients in our whole PCI population, which is generally over 50%. This fact and the exclusion of patients in cardiogenic shock led to a relatively low rate of MACE and major bleeding (primary endpoint). However our pre-defined secondary endpoint was chosen to be relevant to everyday practice, where minor bleeding complications are not without significance. While not being immediately life threatening, minor bleeding may affect prognosis in ACS patients2. Similarly, femoral puncture site complications may be painful, pose a major inconvenience to both patient and physician, and may prolong hospital stay.
In this study UFH was given to achieve a goal ACT of 300 seconds, or 250 seconds for patients receiving glycoprotein IIb/IIIa inhibitors. A lower dose, which has been tested for very low risk patients in the CIAO study, could have favourable effects on bleeding 21.
A further issue is the scope for bias inherently present in a single centre open label study. In order to avoid this problem, assessment of endpoints was done by experienced and specially trained quality control nurses, who were unaware of the patients’ treatment assignment during PCI. Treatment decisions which could influence endpoints, such as the use of transfusions or the indication for surgical procedures for management of puncture site complications, were made by physicians blinded to the treatment allocation. It should be emphasised that operators were not involved in the assessment of bleeding events.
Clinical implications
Intravenous enoxaparin compares favourably with the standard treatment with UFH as recommended by guidelines for PCI. Our study adds to the increasing evidence for the efficacy and safety of enoxaparin for all patient groups undergoing PCI9,10,22. The duration of antithrombotic treatment may be of particular importance. Whereas in our study no prolonged antithrombin treatment was given, in the OASIS-5 study prolonged enoxaparin treatment over a mean of six days resulted in a increased rate of bleeding compared to fondaparinux in patients with ACS23. Recently, bivalirudin has been compared with enoxaparin or UFH in patients with ACS receiving in addition glycoprotein IIb/IIIa inhibitors24. In this setting bivalirudin monotherapy was associated with significantly lower rates of bleeding. It is noteworthy to realise that enoxaparin was administered to many patients subcutaneously and intravenously. Antithrombotic treatment could be continued at the discretion of the operator. As to our best knowledge, there is no study comparing the simple enoxaparin dosage we used in our study compared to bivalirudin in PCI.
However, due to the early termination and the lower than expected event rate no definite conclusion could be made concerning efficacy and safety of intravenous enoxaparin as an alternative to UFH in unselected patients undergoing PCI. Therefore, additional studies are necessary to prove the findings within this trial. Further issues also need to be addressed in the future. In contrast to UFH, which can be antagonised by protamin, the most effective way to antagonise enoxaparin is not clear. This is an important problem in patients with an increased risk of active bleeding, and in patients with an increased risk of coronary perforation such as PCI in chronic total occlusions or use of rotablation. Furthermore the economic consequences of routine use of enoxaparin for coronary interventions are not yet studied and need to be compared with other antithrombotic regimens.
Acknowledgements
All patients were recruited at the Triemli Hospital.
The investigators would like to thank Prof. H.P. Brunner-La Rocca for his advice in preparing the manuscript, Dr. D. Spirk for logistical and organisational support of this project and the members of the data monitoring and safety committee: Prof. F.W. Amann, Prof. W. Kiowski, Dr. A. Vuilliomenet.

