Abstract
Aims: Patients with symptomatic chronic total occlusions (CTO) remain a therapeutic challenge. Enhancement of intraluminal neovascularisation by pro-angiogenic therapies has been proposed as a new strategy to improve percutaneous revascularisation. The aim of this study was to investigate the effects of intraluminal injection of bone marrow-derived cells (BMC) into experimental CTO.
Methods and results: CTO were created in the femoral arteries of 43 New Zealand White rabbits using the thrombin injection model. At 12 weeks following CTO creation, 33 rabbits were injected with either cultured BMC (n=19) or control DMEM alone (n=14) directly into the CTO. Ten rabbits were used for cell tracking (seven BMC and three control). BMC labelled with fluorescent Qdot® nanocrystals were identified in the CTO up to one week after injection. Animals were sacrificed at three to five weeks post-treatment and arterial samples were excised for micro-CT imaging and histologic morphometric analysis. There was a significant but modest increase in neovascularisation in BMC-treated arteries compared to controls (7.47±4.75% vs. 4.35±2.97%, p<0.05). However, unexpected intravascular calcification was only detected within the CTO in BMC cell treated arteries. Western blot for conditioned medium from BMC showed up-regulation of osteogenic proteins (BMP-2 and -7).
Conclusions: Although direct delivery of BMC into CTO increases neovascularisation, undesirable vascular calcification will limit this therapeutic approach.
Introduction
Chronic total occlusions (CTO) are reported in approximately 20% of patients with coronary artery disease who undergo coronary angiography. Despite significant symptoms in a large number of these patients, percutaneous coronary intervention (PCI) is attempted in a small proportion of CTO patients1. The reluctance is frequently due to an anticipated procedure failure related to unsuccessful guidewire crossing. Previous studies by our group have indicated an intricate maturation process within an occluded artery that begins as a thrombus-laden channel in the initial phase, and then progresses to a proteoglycan-rich matrix with abundant microvessels at six weeks, and later to a collagen-rich, poorly vascularised matrix at three months and beyond2. Therapeutic angiogenesis achieved by local delivery of an angiogenic agent has been raised as a treatment option to facilitate guidewire crossing, either by providing small channels for the guidewire to traverse the CTO or by softening the occlusion and altering tissue compliance3-6. We previously reported that local delivery of VEGF164 protein into CTO increased microvessel formation and facilitated guidewire crossing across the proximal fibrous cap7. In the current study, we hypothesised that an alternative angiogenic approach, injection of bone marrow-derived cells (BMC) directly into CTO, would result in an even more enhanced intravascular neovascular response. Thus, the aim of this study was to investigate the effects of intraluminal injection of BMC in an experimental CTO model.
Materials and methods
IN VITRO
BONE MARROW PREPARATION AND CELL CULTURE
Bone marrow (3 to 5 ml) was aspirated from rabbit iliac crest and collected in heparin blood collection tubes containing 2 ml of DMEM (Invitrogen, Life Technologies, Grand Island, NY, USA) supplemented with antibiotics and 10% fetal bovine serum (FBS). Bone marrow samples were filtered through a pre-wet 70-µm cell strainer and the filtrate was centrifuged (400×g, 5 min, 22°C). The pellets containing BMC were washed four times with PBS containing antibiotics and 2% FBS. To get a sufficient cell number and support cell differentiation toward the endothelial cell lineage, cells were cultured on either fibronectin-coated culture dishes (Cat# CACB-356009; BD Biosciences, Franklin Lakes, NJ, USA) according to a modification of Iwase et al8, or on cover slides, in endothelial growth medium-2 microvascular (EGM-2MV; Cat# CC-4147; Lonza, Walkersville, MD, USA). EGM-2MV consists of endothelial basal medium-2, 5% fetal bovine serum (FBS), and supplemental growth factors, vascular endothelial growth factor (VEGF), basic fibroblast growth factor, epidermal growth factor, and insulin-like growth factor-1. After four days, non-adherent haematopoietic cells were removed and fresh EGM-2 was added to the adherent BMC.
For CTO injections, cell layers grown for seven days were dissociated from culture dishes using StemPro® Accutase® (Cat# A11105-01; Invitrogen, Life Technologies), washed three times with ice-cold PBS and suspended in 500 µl of DMEM without supplements. Cells were kept on ice until injection.
CHARACTERISATION OF BMC
Characterisation of BMC cultured on fibronectin was done on day seven, the time point at which the cells were injected into CTO. For immunostaining, after paraformaldehyde fixation, cells were incubated with 1:10 dilution of monoclonal antibodies CD34 (Cat# M1636; Sanquin, Amsterdam, The Netherlands), CD31 (Cat# JC70A; Dako, Glostrup, Denmark), CD146 (Cat# MAB16985H; Millipore, Billerica, MA, USA), CD14 (Cat# MRB1430; Antigenix America Inc., Melville, NY, USA), RAM-11 (Cat# M0633; Dako), CD45 (Cat# MRB4520; Antigenix), 1:50 dilution of eNOS and caveolin-1 (Cat# 610057; BD Transduction Laboratories, Franklin Lakes, NJ, USA), 1:40 dilution of vitronectin receptor, and 1:200 dilution of osteonectin (Cat# M125; Takara Bio Inc., Otsu, Shiga, Japan) at 4°C, overnight. Slides were incubated with 1:50 dilution of Alexa Fluor 488 rabbit anti-mouse IgG (Cat# A11059; Invitrogen, Life Technologies), at room temperature for one hour. All stains were counterstained with DAPI (1 µg/ml) and mounted with fluorescent mounting media (Cat# S3023; Dako). Functional characteristics of cultured BMC were assessed by acLDL uptake Dil-acLDL (molecular probes), and in vitro angiogenesis using Matrigel (Millipore), according to the manufacturer’s protocol9. Flow cytometric analysis was also performed to detect the expression of CD31, CD34 and CD146 as markers for endothelial cell lineage.
WESTERN BLOT ANALYSIS OF CONDITIONED MEDIUM
To identify angiogenic and osteogenic proteins, western blotting was performed. After seven days in culture, cells were washed three times with PBS and incubated in serum-free basal medium (EBM-2 Clonetics; Cat# CC-3156; Lonza) for four days. Conditioned medium was collected, mixed with an equal volume of 20% TCA, and kept at 4°C overnight to precipitate proteins. The precipitate was washed three times with 100% ethanol and dissolved in warm (65°C) SDS sample loading buffer. Aliquots corresponding to 15 µg of cell protein were analysed by 4-20% SDS-PAGE and electrotransferred onto nitrocellulose membranes. Membranes were immunoblotted with rabbit anti BMP-2 (Cat# ARP45066; Aviva Systems Biology Corp., San Diego, CA, USA) and rabbit anti BMP-7 (Cat# AV32329; Sigma-Aldrich, St. Louis, MO, USA) antibodies. Anti rabbit IgG-HRP secondary antibody (Cat# SC-2313; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for detection of primary antibodies and revealed using chemiluminescence detection system followed by autoradiography using BioMax film.
MINERALISATION ASSAY
To evaluate calcium deposits by BMC in one-week and two-week cultures, Alizarin Red S (ARS) staining was performed10.
IN VIVO CTO MODEL
The in vivo study protocol was approved by the Animal Care Committee at Sunnybrook Health Sciences Centre. Arterial occlusions were created in 43 male New Zealand White rabbits (Charles River Canada, St. Constant, Quebec, Canada) fed a normal rabbit diet, by injection of thrombin solution (200 IU; Cat# 82-036-3; Millipore, Kankakee, IL, USA) into a femoral artery segment, as previously described7. The distal ligature was maintained for 60 minutes to ensure a persistent occlusion.
IN VIVO STUDIES
At 12 weeks following CTO creation, 33 rabbits were anaesthetised and injected with either the unselected cultured BMC (n=19) or DMEM media alone (n=14) directly into the CTO.
Rabbits were treated with heparin (1,200 USP units; Pharmaceutical Partners of Canada Inc., Richmond Hill, ON, Canada). A 5 Fr MB1 guiding catheter (Medtronic Inc., Minneapolis, MN, USA) was advanced into the distal abdominal aorta through a 5 Fr sheath placed in the right common carotid artery. Under fluoroscopic guidance, a FineCross microcatheter (Terumo, Somerset, NJ, USA) was positioned directly in contact with the proximal part of the CTO. After removal of the guidewire, BMC (median 3.3×106, 25th percentile 1.3×106; 75th percentile 4.5×106) in 500 µl DMEM media were delivered through a FineCross microcatheter (Terumo). The injection was done slowly over three to five minutes. The microcatheter was then flushed with an additional 500 µl of DMEM medium, and left in place for 30-40 minutes prior to removal. The rabbits were recovered and returned to the cage for an additional three to five weeks (i.e., 15-17 weeks since the CTO was created). Since there were no significant intergroup differences between three-week (n=21) and five-week (n=12) groups, the results were pooled. All of the animals had CTO confirmation by angiography. Following sacrifice, CTO specimens were collected, fixed in 10% neutral buffered formalin, embedded in paraffin, stained with haematoxylin/eosin, and the samples were examined under light microscopy.
In a separate series of experiments to track injected cells within the CTOs, BMC were labelled with red-fluorescent Qdot® 655 nanocrystals (Cat# Q25021MP; Invitrogen, Life Technologies) before injection, according to the manufacturer’s protocol (n=7). Control CTOs were injected with DMEM medium (n=3). CTO injections were performed as described above. We injected labelled BMC to seven CTO rabbits and sacrificed them at four hours (n=3), 24 hours (n=1) and one week (n=3) after cell injection. Control CTO rabbits were sacrificed at four hours (n=2) and at one week (n=1) after cell injection. CTO specimens were embedded in OCT, snap frozen in liquid nitrogen and stored at –80°C until use. Samples were then examined under fluorescence microscopy for labelled cells.
MICRO-COMPUTED TOMOGRAPHY
Micro-computed tomography was performed on excised arteries in an ex vivo set-up, as previously described7,11. Following sacrifice, a 5 Fr arterial sheath was positioned in the abdominal aorta. After flushing with normal saline, Microfil® (Flowtech Inc., Carver, MA, USA) was injected to fill the vascular structures and form an elastomeric gel, creating a three-dimensional cast of the vasculature within 60 minutes. Femoral arteries were excised within the surrounding muscles and specimens were fixed in 10% neutral buffered formalin. Specimens were embedded in 4% of Difco™ Agar (BD Diagnostics, Franklin Lakes, NJ, USA) and placed into 40 ml clear plastic tubes. This preparation was scanned using GE Healthcare eXplore Locus SP (GE Healthcare, London, ON, Canada) at a spatial resolution of 14 μm. A 3D volume image was reconstructed from the stack of x-ray microtomographic cross-sections normal to the axis of rotation using the GE MicroView software package. Image post-processing and 3D volume rendering comprised several sequential steps. A 3D volume image was reviewed in the GE MicroView software to define the region of interest with subsequent conversion into PNG format by using the MRIcro software package (University of South Carolina, Columbia, SC, USA). The 3D volume rendering and image quantification were performed in Amira® 3D visualisation software for Windows® (Visage Imaging Inc., Andover, MA, USA).
The relative blood volume index (RBVI) in micro-CT was determined as previously described7,11. Specifically, RBV for micro-CT is the ratio of signal intensity from an ROI in the proximal part of the CTO relative to that from an ROI in a patent vessel immediately adjacent to the CTO.
HISTOLOGY ANALYSIS
The arterial specimens were cut into five segments, each of 3-4 mm length. In paraffin embedded specimens, 5 μm-thick cross-sections of the femoral artery were stained with haematoxylin/eosin and Movat’s pentachrome, and Von Kossa stains. Serial slides were analysed to determine the most proximal segment of the CTO to identify the origin of the CTO and the location of the proximal fibrous cap. Morphometric analysis was performed in the most proximal section of the CTO to determine the overall size of the artery (defined by the external elastic lamina), the number of microchannels per section, and the total microvessel area within the vessel, i.e., the combined medial and intraluminal microvessel area as defined by the border of the elastic lamina externa, as previously described7. The percent of microvascular area was calculated as the percent of total microvessel area relative to the overall vessel size. Calcification was assessed in Von Kossa stained cross-sections.
STATISTICS
Data are presented as continuous variables and expressed as mean±SD. Categorical variables data are expressed in frequencies (%). For comparisons of the data, analyses of variance (ANOVA) with a Student’s t-test were performed where the F-test among groups was statistically significant. Statistical analysis was done with the Statistical Package for Social Science (SPSS) software version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
Results
IN VITRO RESULTS
CHARACTERISATION OF BONE MARROW-DERIVED CELLS
Cells cultured on fibronectin for seven days were examined. Light microscopy examination showed that the BMC were morphologically heterogeneous and consisted of round cells and spindle-shaped cells and formed cord-like and luminal structures. At the end of the first week, or beginning of the second week, dotted cells appeared and their population increased with time. At one month, circular structures appeared.
Fluorescence microscopy showed that the cultured BMC expressed CD34 (Figure 1A), CD31 (Figure 1B), CD146 (Figure 1C) and eNOS (Figure 1D), all markers of endothelial lineage cells. Moreover, the cells expressed caveolin-1, a protein known to associate with eNOS in mature ECs (Figure 1E). BMC took up acLDL (Figure 1F) and rapidly formed network structures in Matrigel in four hours (Figure 1G), indicating functional characteristics of ECs. In addition, some cells stained positive for CD14, a monocyte marker (Figure 1H), and for RAM-11, a macrophage marker (Figure 1I). Flow cytometric analysis showed positive staining for CD31 (0.55%), CD34 (8.31%), CD146 (11.4%), CD45 (1.04%) and vitronectin receptor (20.5%).
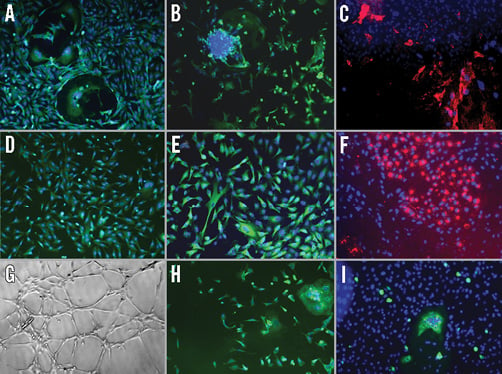
Figure 1. Characterisation of cultured bone marrow-derived cell (BMC) at one week, the time point at which cells were injected into CTO. A) Cells stained positive for CD34 (EPC marker). B) CD31 (EC marker). C) CD146 (red, EC marker). D) eNOS (EC marker). E) Caveolin-1 (EC marker). F) Cells took up acLDL (red), demonstrating functional characteristics of EC. G) Cells formed capillary networks in Matrigel demonstrating functional characteristics of EC. H) Cells stained positive for CD14 (monocyte marker). I) RAM-11 (green, macrophage marker). EC: endothelial cell; EPC: endothelial progenitor cell
IN VIVO STUDIES
BMC TRACKING STUDY
Red-fluorescent Qdot® 655 nanocrystal labelled BMC were identified by fluorescence microscopy within all the CTO at the three time points (four hours, 24 hours and one week) (Figure 2). Control arteries injected with medium alone were negative for Odot nanocrystal immunofluorescence, indicating the specificity of the method. These studies indicate that the BMC were identifiable within the CTO for at least one week following the injection of the cells.
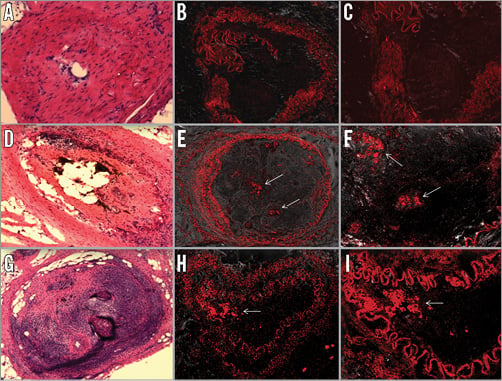
Figure 2. Tracking of BMC into CTO using Qdot nanocrystals to confirm delivery and residency of BMC. The cells were labelled with Qdot® 655 nanocrystal and injected into CTO. Rabbits were sacrificed at four hours and one week and the samples were analysed by fluorescence microscopy. A)-C) Control-Medium. H &E staining (A). Qtracker labelled cells (×10) (B). Qtracker labelled cells (×20) (C). D)-F) Labelled cells 4 hours after injection. H &E staining (D). Labelled cells (×10) (E). Qtracker labelled cells (×20) (F). G)-I) Labelled cells 1 week after injection. H &E staining (G). Labelled cells (×10) (H). Labelled cells (×20) (I). White arrows indicate the cells labelled with Qdot® 655 nanocrystal (red).
MICRO-CT ANGIOGRAPHY AND HISTOLOGY
There was a significant increase in neovascularisation within the occluded lumen in cell-treated CTOs as determined by both micro-CT and histology (Figure 3). The blood volume index determined by micro-CT showed a significant increase in BMC-treated arteries compared to controls (7.47±4.75% vs. 4.35±2.97%, p<0.05). However, an unexpected finding was the presence of calcification within the CTO in seven of 19 (37%) BMC-treated arteries (Figure 4A and Figure 4B). No calcifications were evident in any of the control arteries.
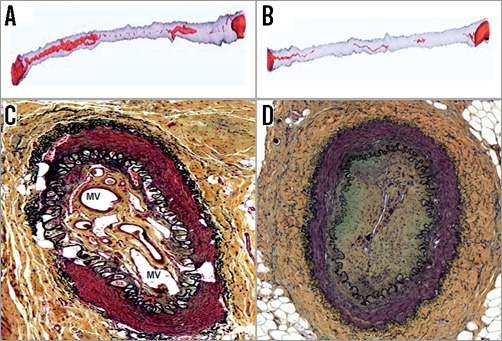
Figure 3. Volumetric images obtained from micro-CT analysis show increased intraluminal neovascularisation in BMC-treated CTO which was confirmed by histology results. A) Micro-CT analysis of BMC-treated CTO. B) Micro-CT analysis of control group. Histology showed increased microvessels in BMC-treated CTO. C) Histology of BMC-treated CTO. D) Histology of control group. MV: microvessel
OSTEOGENIC AND MINERALISATION STUDIES
Cultured BMC at one week were negative for Alizarin Red S staining, but positive red staining was present in two-week-old cultures, indicating mineralisation (Figure 4C and Figure 4D). At one week, cultured BMC stained positive for vitronectin receptor (Figure 4E), an osteoclast marker, but negative for osteonectin, an osteoblast marker. However, at two weeks cultured BMC cells stained negative for vitronectin receptor but positive for osteonectin (Figure 4F). There was no correlation between delivered BMC number and microvessel area or ossified area (Figure 5). At one week, osteogenic factors BMP-2 and BMP-7 were present in western blot from BMC conditioned medium (Figure 6).
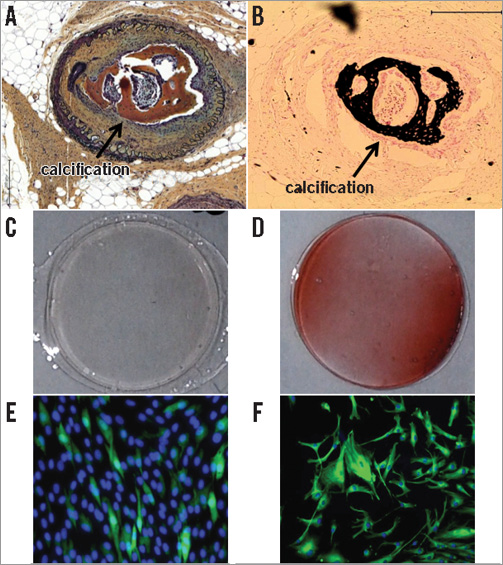
Figure 4. Calcification was evident in seven of 19 BMC-treated CTO. A) Movat stain. Calcified area is brown. B) Von Kossa stain. Calcified area is black. C) & D) Alzafarin Red staining for calcium deposits. At 1 week of cell culture, negative staining for Alzafarin Red (C). At 2 weeks of cell culture, positive red staining for Alzafarin Red (D). E) & F) Mineralisation and osteogenic protein expression in vitro. At 1 week of cell culture, osteoclast markers were identified (green) and no calcification (E). At 2 weeks of cell culture, osteoblast markers were identified (green), osteoclast markers were absent and calcification was present (F).
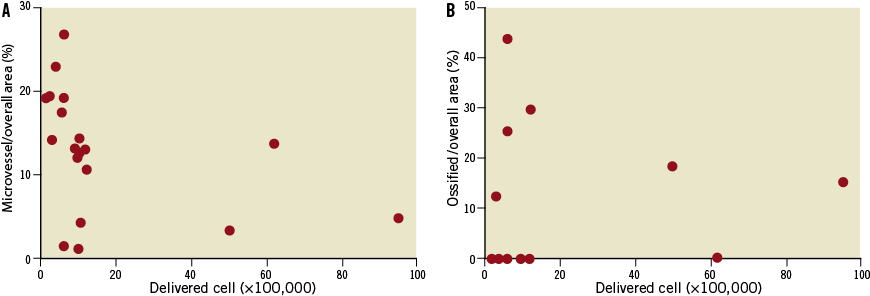
Figure 5. There was no significant correlation between delivered cell number and microvessel area or ossified area. A) Correlation between delivered cell number and microvessel area/overall area (%). B) Correlation between delivered cell number and ossified area/overall area (%).
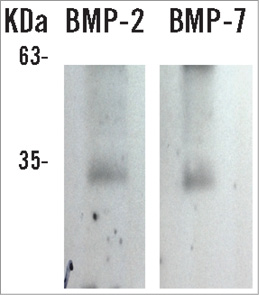
Figure 6. Osteogenic protein expression. BMP-2 and BMP-7 were present in conditioned medium of BMC culture after one week.
Discussion
The major findings of this study were that direct intravascular injection of cultured BMC into CTO showed a significant but modest increase in the neovascularisation response, but also unexpected significant calcification of injected arteries. Our results raise another concern about one of the complications of bone marrow cell transplantation. Numerous studies of unselected or mesenchymal stem cells derived from bone marrow have reported enhanced therapeutic angiogenesis for treating ischaemic heart diseases12-14 or ischaemic limb15, by enhancing collateral development16.
Based on the literature and our own experimental observations of local delivery of VEGF into CTO increasing microvessel formation7, we hypothesised that local injection of BMC directly into CTO would alter the architecture by increasing neovascularisation. Previous studies from our group have suggested that increasing CTO intraluminal neovascularisation may enhance guidewire crossing7. To obtain adequate cell numbers for cell delivery, we cultured cells obtained by bone marrow aspiration. The cultured BMC showed surface markers of endothelial lineage cells by both fluorescence microscopy and flow cytometric analysis. Also, the cultured BMC demonstrated functional characteristics of endothelial lineage cells by taking up acLDL and rapidly formed network structures in Matrigel.
Since this was the first study of BMC effects in CTO, it was not known whether the BMC could penetrate into the CTO and still be evident in the early period after injection. We were reassured by the labelled BMC studies that the cells could be readily identified in the body of the CTO at least one week after the injection. In this model, it is extremely challenging to determine the duration and absolute number of BMC present at different levels of the CTO. Tracking of labelled BMC with red-fluorescent Qdot® 655 nanocrystals indicated that the BMC were localised to the proximal and the mid section of the CTO. The presence of the cells, even at one week post injection, and the biologic effects (angiogenesis and calcification) of the cells suggest that these BMC were functional and active. We consider it highly unlikely that a significant number of BMC entered collaterals (which are typically outside the vessel wall) and re-entered the systemic circulation since we performed a direct injection into the CTO.
Similarly, the optimal cell number for delivery and therapeutic effect is not known. Given the limited amount of bone marrow that we could aspirate, it was necessary to culture the BMC to expand the cell population. After seven days of culture, the cell numbers isolated for injection had some variability (1.3-4.5×106, 25th-75th percentile), but the adequacy of the cell number was supported by a significant increase in both percent blood volume measured by micro-CT and by total microvascular area measured by histology. The increase in blood volume index in the BMC-treated group as determined by micro-CT was similar to our previous study of VEGF protein injection in the same model7.
Despite the positive results of this approach (penetration of the BMC into the body of the CTO and the modest increase in angiogenic response), we also observed significant intravascular calcification. The calcification would probably be clinically significant, based on its overall extent and its location within the occluded lumen. The presence of calcification is a recognised risk factor for failure of PCI revascularisation17. In the current study, there was no correlation between delivered cell number and microvessel area or ossified area. The reasons for the absence of a relationship between cell number and these effects is unknown, but may be related to the relatively narrow range of cell number injected. Alternatively, these effects may be due to a particular subset of cells which was not directly quantified.
The mechanisms by which transferred BMC modulate vascular calcification remain unclear. There was no significant correlation between delivered cell number and ossified area. The first possible mechanism is the influence of the cellular origins of BMC that we delivered. Bone marrow is composed of a mixture of haematopoietic progenitor cells, mesenchymal cells, adipocytes, fibroblastic cells, and osteoblasts18. It has been well established that mesenchymal cells can differentiate into osteoblasts, chondrocytes, adipocytes, vascular cells or cardiomyocytes under special conditions19-22. Although our cultured cells showed endothelial cell lineage markers such as CD31, CD34, CD146, eNos and caveolin-1, other stem cells could be mixed and promote vascular calcification. It is not clear whether endothelial progenitor cell lineage or other mesenchymal stem cells are involved and responsible for calcification in this CTO model.
Second, the possible interaction between the transplanted BMC and the resident vascular cells in the arterial wall cannot be excluded. The outcome of transplanted cells is likely to be influenced by the method of cell isolation, dose, timing of transplantation and clinical setting. There is a possibility that unwanted differentiation may occur after BMC transplantation in a situation prone to be calcified such as our CTO model. In our CTO model, the osteoclast-osteoblast interaction mediated via bone morphogenetic protein (BMP) may be a potent contributing mechanism of vascular calcification. In our in vitro studies, one-week cell cultures were positive for osteoclast markers but negative for osteoblast markers, and there was no evidence of mineralisation. However, at two weeks osteoclast markers were no longer identified but both osteoblast markers and mineralisation were present. Activated osteoclasts produce BMPs, growth factors such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta (TGF-β): these factors might stimulate differentiation and activation of osteoblasts23,24. In addition to this osteoclast-osteoblast interaction, BMP-2 and BMP-7 may promote vascular calcification via increased phosphate uptake and induction of osteogenic phenotype modulation in smooth muscle cells25,26, and by promoting proliferation and differentiation of undifferentiated BMC to cartilage and bone27. The relationship between BMC-mediated calcification and interactions with osteoclasts, osteoblasts and BMP merit further investigation.
Indeed, concerns have been raised previously about the potential adverse effects of stem cell transplantation in vivo28-30. Injection of mononuclear cells isolated from mouse bone marrow into peri-infarct zones of myocardium has resulted in the development of malignant sarcomas28. Intramyocardial calcification has been reported following injection of unselected bone marrow stem cells in mice29. Recently, Liao and colleagues reported vascular calcification in the media of rat aortic arteries at six weeks following balloon injury and injection with bone marrow-derived mesenchymal stem cells30. Our results provide another cautionary example of the potential toxicity of therapeutic applications of cell transplantation in the vasculature.
Study limitations
Since stem/stromal cell lines and stem cell lines are not identical, their characterisation and function can vary depending on their origin and isolation method. Further investigation is required to determine whether one should use unstimulated bone marrow-derived cells or a more differentiated cell line for neovascularisation or regeneration of a tissue. Our results cannot be generalised to other specific cell lines.
A potential concern of this therapeutic approach is that neovascularisation within the CTO could promote plaque destabilisation. Preclinical and clinical human coronary studies have identified plaque neovascularisation as an important factor in the generation of vulnerable plaque leading to acute myocardial infarction31. It is not known whether this could also occur in chronic total occlusions which have been successfully revascularised. Thus, the long-term effects of inducing an angiogenic response on a chronic total occlusion, such as predisposing to plaque rupture and acute coronary syndromes or to stent thrombosis, merits further study.
Conclusion
In summary, although cultured BMC expressed cell surface markers of angiogenic cell types, secreted angiogenic proteins into conditioned medium and showed significantly increased in vivo neovascularisation response, injection of these BMC was complicated by the appearance of undesirable extracellular calcifications in an experimental CTO model. This study indicates that caution should be exercised when using cultured BMC for angiogenic effects in CTO.
| Impact on daily practice Chronic total occlusions (CTO) remain a complex group of lesions to revascularise by PCI. Intraluminal microvessels form during early stages of CTO formation and may facilitate PCI, but regress over time as the CTO matures. A strategy to increase intraluminal neovascularisation by injection of bone marrow-derived cells with angiogenic markers into mature CTO resulted in a modest increase in microvessels, but unwanted intraluminal calcification limits the potential clinical utility of this therapeutic strategy. |
Funding
Supported by the Canadian Institute of Health Research (grant number MOP - 53325; grant number CTP 82943).
Conflict of interest statement
B.H. Strauss holds a patent for the use of angiogenic therapy, including bone marrow-derived cells, in chronic total occlusions. The other authors have no conflicts of interest to declare.

