Abstract
Aims: Percutaneous revascularisation of chronic total occlusions (CTO) is limited by failure of guidewire crossing. Neovascularisation within the proximal CTO segment may be important for guidewire crossing and dramatically declines in CTO beyond six weeks of age. The aims of the current study were to determine whether local delivery of a pro-angiogenic growth factor increases neovascularisation in mature CTO and facilitates guidewire crossings.
Methods and results: CTO (n=51) were created in the femoral arteries of 44 New Zealand white rabbits using the thrombin injection model. At 12 weeks, CTO were treated with poly-lactic-glycolic-acid (PLGA) microspheres containing either bovine serum albumin (BSA) (n=15) or recombinant mouse VEGF164 (n=14), or received no intervention (controls, n=12). Contrast-enhanced magnetic resonance angiography (CEMRA) was performed prior to treatment and at three weeks post treatment. Animals were sacrificed at three weeks post treatment and arterial samples were excised for micro-computed tomography imaging (µCT) and histologic morphometric analysis. Guidewire crossing was assessed at three weeks post treatment in an additional 10 VEGF164-treated CTO. In comparison to BSA-treated and control non-intervened CTO, VEGF164-treated CTO showed a significant increase in relative blood volume index in the proximal segment of the CTO lesion as determined by CEMRA and by µCT. Histologic measurements of microvessel area were also higher in VEGF164-treated CTO. Guidewire crossing across the proximal fibrous cap was successful in eight out of 10 VEGF164-treated CTO.
Conclusions: Angiogenic therapy appears to be a promising strategy to improve neovascularisation and guidewire crossing rates in CTO.
Introduction
Percutaneous coronary interventions (PCI) in chronic total occlusions (CTO) remain a challenge for interventional cardiologists. Despite advances in techniques and equipment, PCI success rates for CTO have not increased over the last five years1,2. Underlying these high failure rates is a lack of appreciation of the underlying composition of the CTO. We have recently reported on the temporal changes in CTO composition in an experimental model3. We observed transient neovascularisation in the proximal segment of the CTO that peaked at six weeks after occlusion formation, but then progressively regressed over the ensuing weeks. Clinical experience has indicated that success rates are high in occluded arteries for up to six weeks, but then dramatically decline at later time points, a time frame that closely parallels the formation and then regression of the intraluminal microvascular network4. This reduction in microvessels is particularly important at the site of the proximal fibrous cap, which acts as an occlusive barrier to initial passage of the guidewire into the CTO3. More recently, Carlino et al have shown that injection of contrast directly into CTO facilitates guidewire crossing in selected CTO, potentially through enlargement of pre-existing microchannels5-7.
We hypothesised that local delivery of a potent angiogenic agent directly into older, poorly vascularised CTO could recreate the robust network of microchannels seen at earlier time points, particularly at the proximal fibrous cap, that could facilitate guidewire crossing. In this report, we describe our in vitro and in vivo results with local injection of VEGF164 (R&D Systems Inc., Minneapolis, MN, USA) protein into experimental CTO.
Methods
MICROSPHERE SYNTHESIS
Biodegradable PLGA microspheres were fabricated using a double emulsion technique (water-oil-water) as described previously8-10. A 5% PLGA solution in chloroform was vortexed at constant speed in the presence of 1% polyvinyl acetate (PVA) solution in 7.5% dextrose to emulgate the polymer and allow microsphere formation. Scanning electron microscopy confirmed the microsphere mean size of 20 µ in diameter (Figure 1). A suspension was frozen at –80°C and lyophilised. The lyophilised microspheres were stored at –20°C until needed.
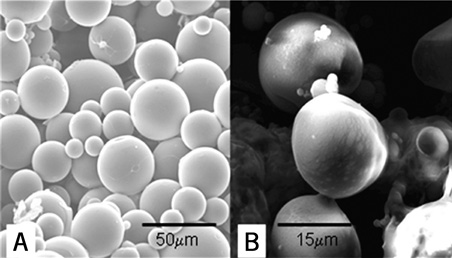
Figure 1. Scanning electron microscopy of the biodegradable polylactic-glycolic-acid microspheres. A) ×600, bar=50 μm. B) ×2000, bar=15 μm
Bovine serum albumin (BSA) has an important function to open pores within PLGA microspheres to ensure egress of the agent from within the interior of the microsphere. Microspheres were prepared with either BSA alone or BSA with recombinant mouse VEGF164. The BSA and BSA/ VEGF164 microspheres were prepared by incorporation of either 10 mg of BSA or 10 mg of BSA plus 5 µg of VEGF164 per batch, with each batch making approximately five treatments.
VEGF RELEASE PROFILE IN VITRO
The in vitro VEGF164 release profile from PLGA microspheres was determined using the Quantikine® mouse VEGF immunoassay kit (R&D Systems Cat#MMV00). Briefly, 18 mg of PLGA microspheres containing ≈1 µg VEGF164 were reconstituted in phosphate buffers and aliquoted into FalconTM conical tubes (BD Biosciences, San Jose, CA, USA). At predetermined time points, samples were centrifuged at 2,000 rpm for 10 minutes at room temperature and the 2 ml supernatant collected and stored at –20˚C until analysis. The cumulative amount of VEGF164 released from the microspheres over 167 hours was measured according to immunoassay kit protocol. The experiment was repeated in five separate samples.
MICROSPHERE VEGF BIOACTIVITY POST-RELEASE
The bioactivity of the VEGF164 incorporated into, and released from, the biodegradable microspheres was determined by testing its ability to stimulate growth in a cultured mouse endothelial cell line (MS1). MS1 cells were cultured to passage in Dulbecco’s modified eagle’s medium (DMEM) (HyClone Cat #SH30022.01; Thermo Scientific, Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS) (Cat#SH3039603C; Fisher Scientific, Loughborough, UK) and 1% penicillin-streptomycin (Cat#450-201-EL; Wisent Inc., St-Bruno, Quebec, Canada). Cells were plated at a density of 3×103 cells per well on 96-well flat-bottom tissue culture plates (Cat#83.1835; Sarstedt Inc., Newton, NC, USA) in 100 µl 0.25% FBS in DMEM. Cells were serum-starved for 24 hours at 37˚C, 5% CO2, and then placed in 0.25% of FBS/DMEM supplemented with the following treatments for 24 hours: 0.1 ng VEGF164, and VEGF164 that had been released from microspheres, as well as positive control (10% FBS/DMEM) and negative control (DMEM alone). We collected 100 microlitres of supernatant containing VEGF164 released from the microspheres over one hour as described above. The VEGF164 concentration in the supernatant at this time was 0.995 ng/ml, so approximately 0.1 ng in total would have been added. The WST-1 proliferation reagent (Cat#11 644 808 001; Roche Diagnostics, Hoffmann-La Roche, Basel, Switzerland) was then added and the plate was incubated for one hr. Endothelial cell growth was measured by absorbance at 450 nm using the PowerwaveTM X340-1 plate reader (BioTek Instruments Inc., Winooski, VT, USA). Experiments were done in triplicate.
CTO MODEL
The experiments were done in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) Sunnybrook and Women’s Research Institute A5176-01. The in vivo studies were approved by the Animal Care Committee at Sunnybrook Health Sciences Center. Surgical procedures on rabbits were performed under general anaesthesia. For induction, the mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg) was administered intramuscularly in hind extremities. During surgery, general anaesthesia was maintained via inhalation of the gaseous mixture of isoflurane (1-3 vol%) and oxygen (2.5-3 L/min). For monitoring, a pulse oximeter was attached to the paw to monitor heart rate and oxygen saturation in order to assess depth of anaesthesia properly. Rabbits were tracheally intubated with a 3 Fr endotracheal tube and maintained at a surgical plane of anaesthesia through the delivery of inhalant isofluorane (2-3.5%) with 2-3 l/min of oxygen. Immediately after surgery, rabbits were placed for recovery into neonatal incubators with an environmental temperature of 32-35°C. Euthanasia via overdose of sodium pentobarbital (240 mg/ml dosage 3 ml/3 kg) was delivered through an intravenous (IV) catheter placed in the marginal ear vein of the anaesthetised rabbit, which outlines the humane method used to avoid additional animal pain and suffering. This was preceded by a general anaesthesia (ketamine [50 mg/kg] and xylazine [5 mg/kg] intramuscular) administration of Euthanyl (pentobarbital sodium) (Bimeda MTC Animal Health Inc., Cambridge, ON, Canada) intravenously into the marginal rabbit’s ear.
Arterial occlusions (n=51) were created in 44 male New Zealand white rabbits (Charles River Laboratories, St. Constant, Quebec, Canada), fed a normal rabbit diet by injection of thrombin solution (100 IU) (Millipore cat. no. 82-036-3; Millipore Inc., Kankakee, IL, USA) into a femoral artery segment, as previously described3,11. The distal ligature was maintained for 60 minutes to ensure a persistent occlusion.
INTRAVASCULAR INTERVENTION
At 12 weeks, rabbit CTO were treated by endovascular delivery of PLGA microspheres containing either BSA (n=15) or BSA/VEGF164 (n=14). A third group of CTO rabbits (n=12) did not undergo an intravascular intervention and served as controls. Rabbits were treated with heparin (1,200 USP units; Pharmaceutical Partners of Canada Inc., Richmond Hill, ON, Canada). A 5 Fr MB1 guiding catheter (Medtronic Inc., Minneapolis, MN, USA) was advanced into the distal abdominal aorta through a 5 Fr sheath placed in the right common carotid artery. Under fluoroscopic guidance, a 2.00 mm diameter over-the-wire balloon (Voyager OTW; Abbott Vascular, Abbott Park, IL, USA) was positioned immediately proximal to the CTO and inflated at eight atmospheres to maintain position and prevent backflow of the infusate. After removal of the guidewire, a suspension of 16 mg of biodegradable microspheres in 1 ml of normal saline was gently injected through the wire port into the CTO. Balloon inflation was maintained for 30 minutes, followed by removal of the balloon catheter and arterial sheath. The rabbit was recovered and returned to the cage for an additional three weeks (i.e., 15 weeks since the CTO was created). Rabbits were sacrificed at the 15-week time point.
ASSESSMENT OF MICROVESSEL FORMATION
Multiple complementary methods were used to assess blood flow and microvessel formation as follows:
CONTRAST-ENHANCED MAGNETIC RESONANCE ANGIOGRAPHY (CEMRA)
CEMRA was performed at 12 weeks of CTO (just prior to the interventional procedure), and at 15 weeks (three weeks after the intervention) to determine blood volume in the proximal segment of CTO, as an indirect measurement of flow and neovascular formation. CEMRA was done in a 3.0TTM GE Signa EXCITE® magnet (GE Healthcare, Milwaukee, WI, USA), as previously described3. Briefly, the angiogram was obtained by using a custom 3 cm rectangular surface coil. The following parameters were used for MRI imaging: 3-D spoiled gradient recalled echo (SPGR); field of view 8.0 cm; matrix: 320×320; slice thickness: 0.8 mm; repetition time: TR: 8.4 ms; echo time: 3.3 ms; flip angle: 30 degrees; bandwidth: 31.25 kHz; number of averages: 5; number of slices: 30; imaging time: 6 min. Clariscan™ (dose 0.05 ml/kg, Feruglose; GE Healthcare, Mississauga, ON, Canada), an intravascular iron-based contrast agent with large molecular size, was injected intravenously ~20 seconds before the acquisition of T1 weighted images with the SPGR sequence.
Relative blood volume index (RBVI) was determined from the ratio of signal intensity determined from the region of interest (ROI) at the proximal part of CTO relative to that from a region of interest in a patent vessel immediately proximal to the CTO, as previously described3. Data analysis was done on the Advantage Workstation (Ver. 4.2_07) with Volume Viewer Plus (Voxtool 5.10.4; GE Healthcare, Milwaukee, WI, USA) and expressed as % blood by volume (the percentage of the tissue volume in the ROI occupied by blood).
MICRO-COMPUTED TOMOGRAPHY (μCT )
Micro-computed tomography (µCT) was performed on excised arteries in an ex vivo set-up, as previously described3,12. Prior to sacrifice, animals were intravenously injected with heparin (2,000 USP units) to prevent post mortem intravascular coagulation. Following sacrifice, a 5 Fr arterial sheath was positioned in the abdominal aorta. After flushing with normal saline, Microfil® (Flowtech Inc., Carver, MA, USA) was injected to fill the vascular structures and form an elastomeric gel creating a three-dimensional cast of the vasculature within 60 minutes. Femoral arteries were excised within the surrounding muscles and specimens were fixed in 10% buffered formalin. Specimens were embedded in 4% of DifcoTM Agar (BD, Franklin Lakes, NJ, USA) and placed into 40 ml clear plastic tubes. This preparation was scanned using GE Healthcare eXplore Locus SP (GE Healthcare, London, ON, Canada) at a spatial resolution of 14 µm. A 3-D volume image was reconstructed from the stack of x-ray microtomographic cross-sections normal to the axis of rotation using the GE MicroView software package. Image post processing and 3-D volume rendering comprised several sequential steps. A 3-D volume image was reviewed in the GE MicroView software to define the region of interest with subsequent conversion into PNG format by using the MRIcro software package (University of South Carolina, Columbia, SC, USA). The 3-D volume rendering and image quantification were performed in Amira® 3-D visualisation software for Windows® (Visage Imaging Inc., Andover, MA, USA).
The relative blood volume index (RBVI) in micro-CT was determined in a manner similar to the calculation from MRI data. Specifically, RBV for micro-CT is the ratio of signal intensity from a ROI in the proximal part of CTO relative to that from a ROI in a patent vessel immediately adjacent to the CTO.
HISTOLOGY ANALYSIS
The arterial specimens were cut into five segments, each of 3-4 mm length. In paraffin embedded specimens, 5 µm-thick cross-sections of the femoral artery were stained with haematoxylin/eosin and Movat’s pentachrome. Serial slides were analysed to determine the most proximal segment of the CTO to identify the origin of the CTO and the location of the proximal fibrous cap. Morphometric analysis was performed in the most proximal section of the CTO to determine the overall size of the artery (defined by the external elastic lamina), the number of microchannels per section, and the total microvessel area within the vessel, i.e., the combined medial and intraluminal microvessel area as defined by the border of the elastic lamina externa3.The percent of microvascular area was calculated as the percent of total microvessel area relative to the overall vessel size.
PILOT IN VIVO GUIDEWIRE CROSSING STUDIES
Guidewire crossing studies were assessed in 10 CTO treated with VEGF164-containing microspheres as described above. The initial guidewire attempts were made with soft tip guidewires (ChoICE® PT Extra Support Guide Wire [Boston Scientific, Natick, MA, USA]; WIZDOM® [Cordis, Johnson & Johnson, Warren, NJ, USA]; HI-TORQUE ADVANCETM [Abbott Vascular, Santa Clara, CA, USA]) and, if necessary, then a stiffer tip guidewire (ASAHI Confianza PRO; Abbott Vascular). Successful crossing was based on angiographic confirmation of free movement of the guidewire in the distal vessel11. Guidewire crossing times were recorded for each CTO with a maximum of 40 minutes allowed for guidewire crossing. Arteries were examined histologically after the crossing attempt.
STATISTICAL ANALYSIS
Data are presented as continuous variables and expressed as mean±SD. Categorical variables data are expressed in frequencies (%). For comparisons of the data, analyses of variance (ANOVA) with a Student’s t-test were performed where the F-test among groups was statistically significant. Statistical analysis was performed using the SAS® v.9.0 (SAS, Cary, NC, USA) software univariate procedure to test data normality and the general linear model to fit and test differences among least squares means. A p-value of <0.05 was considered statistically significant.
Results
IN VITRO
VEGF164 RELEASE
The cumulative mass release of VEGF164 in the medium was approximately 22 ng (Figure 2). The profile trend was a triphasic release pattern, characterised by an initial burst in the first hour, followed by sustained, diffusion-controlled release for the next eight hours. As the VEGF164 release slowed, further release was controlled by polymer degradation. Release reached a plateau level at day four.
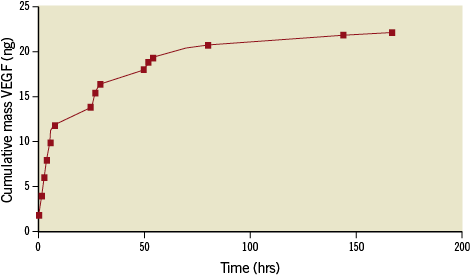
Figure 2. Cumulative in vitro release of VEGF164 from PLGA microspheres over time. The release profile is triphasic, with an initial burst in the first hour, followed by a sustained, diffusion-controlled release for the next 8 hours, leading to a plateau by day 4.
ENDOTHELIAL CELL PROLIFERATION
The VEGF164 released into the medium from PLGA microspheres (a mass of 0.1 ng VEGF164) showed a significant increase in MS1 endothelial cell proliferation compared to 0% FCS/DMEM control (p=0.009, Figure 3), indicating that the released VEGF164 was biologically active. The endothelial cell proliferation effects of the released VEGF164 were similar to 10% foetal calf serum and recombinant mouse VEGF164.
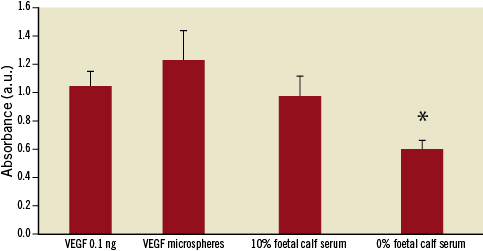
Figure 3. Endothelial cell proliferation. There was a significant increase in proliferation of endothelial cells (MS1 cells) exposed to the supernatant derived from VEGF164 microspheres compared to negative control, DMEM/0% FCS. The proliferative effects of VEGF164 released from the microspheres was similar to VEGF164 0.1 ng and 10% FCS (* p<0.01).
IN VIVO
EFFECT OF INTRAVASCULAR VEGF164-CONTAINING MICROSPHERES INJECTION ON THE CTO BLOOD VOLUME DETERMINED BY CEMRA
At 12 weeks prior to injection of the microspheres, all three groups showed similar values for RBVI in the proximal segment of the CTO. There was a significant increase in RBVI over the three-week period in VEGF164-treated rabbits: VEGF 18.3%±4.8% to 31.6%+4.6% (p=0.001, Figure 4). In contrast, the BSA microspheres and the non-interventional group did not show differences between the two time periods (Figure 4 and Figure 5). At 15 weeks, the RBVI values in VEGF164-treated rabbits were significantly higher compared to either BSA microspheres or the non-interventional group (p<0.006).
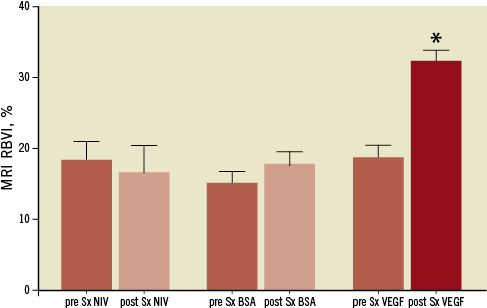
Figure 4. Relative blood volume index determined by Clariscan enhanced MRA prior to microsphere delivery (open bars) and at three weeks post-procedure (dark bars). VEGF164 microsphere-treated arteries at 3 weeks showed significantly higher relative blood volume index compared to pre-VEGF and to both BSA microspheres or non-interventional controls (*p=0.001compared to pre-VEGF treatment; p<0.006 compared to BSA-treated and NIV groups).
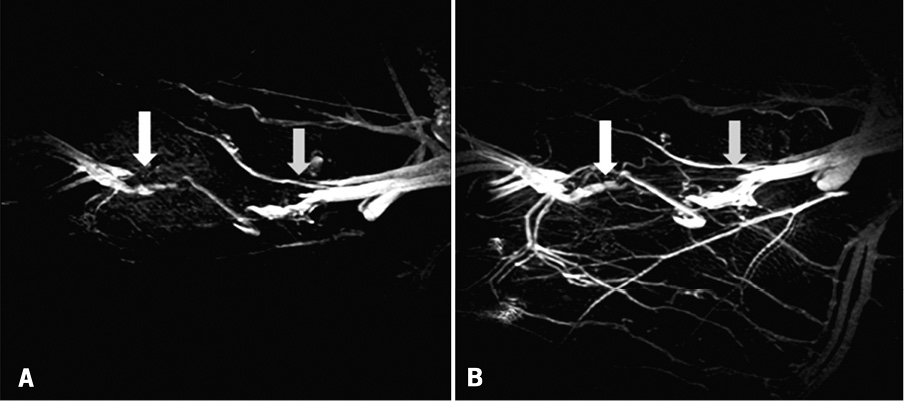
Figure 5. Clariscan enhanced MR images of CTO before (A) and at three weeks (B), following treatment with VEGF164 microspheres. Grey arrow indicates proximal site of CTO and white arrow indicates distal site.
μCT DETERMINED RELATIVE BLOOD VOLUME INDEX
At 15 weeks, VEGF164-treated CTO arteries showed a 4-5 times higher relative blood volume index (4-5x) compared to BSA microsphere-treated and non-interventional groups (p=0.001, Figure 6).
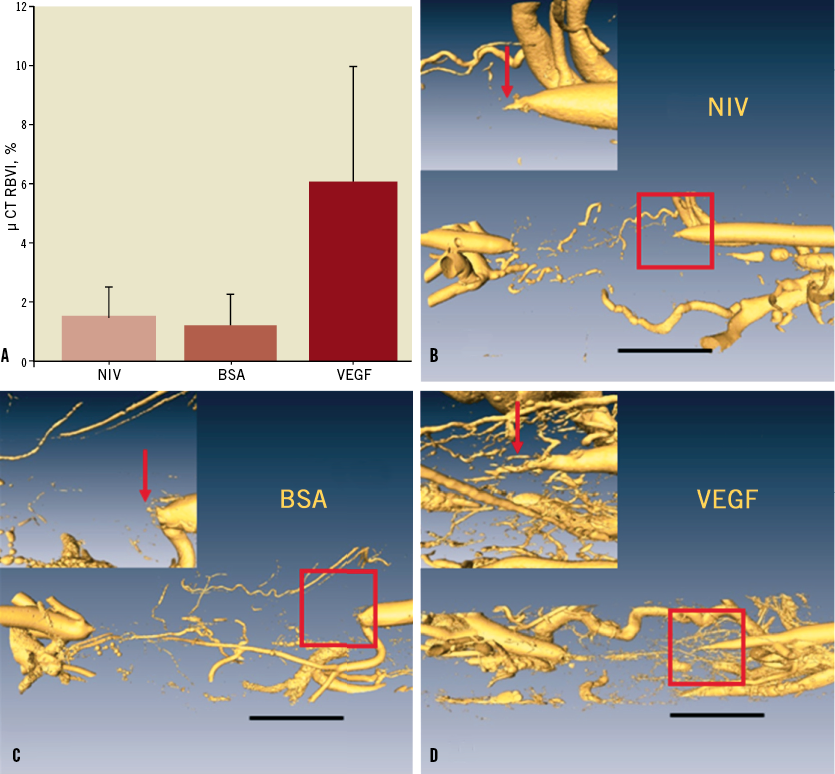
Figure 6. A) Relative blood volume determined by micro-computed tomography. There was a significant increase in VEGF164-treated arteries compared to both BSA microspheres or non-interventional controls. (*p=0.001). B, C, D) Surface-rendered micro-CT data sets for non-interventional control, BSA and VEGF CTO. Resolution equals 14 um; black bar indicates 5mm; the area of interest (outline by red square) is shown in the inset.
HISTOLOGY AND MORPHOLOGIC CHANGES FOLLOWING VEGF164 THERAPY
The occluded lumen contained three principal components: collagen, microvessels and lipid. There was no residual thrombus present in the lumen. The % microvascular area was increased in VEGF164-treated CTO (18.5%±9.3%) compared to BSA-treated (10.5%±6.9%, p=0.07) or non-interventional treated CTO (7.0%±2.2%, p<0.01) (Figure 7, Figure 8A-Figure 8C). In approximately 25% of microsphere-treated arteries, there was evidence of residual microspheres present within the lumen at three weeks (Figure 8D).
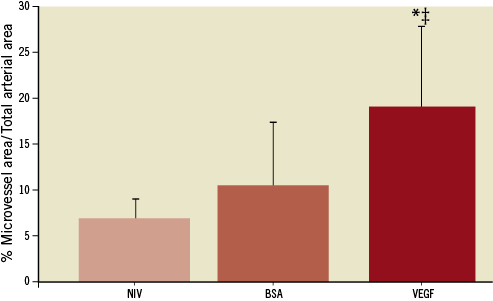
Figure 7. Microvascular area in VEGF164-treated CTO compared to BSA-treated or non-interventional CTO. VEGF164 vs. NIV (*: p=0.01); and VEGF164 vs. BSA; (‡: p=0.07)
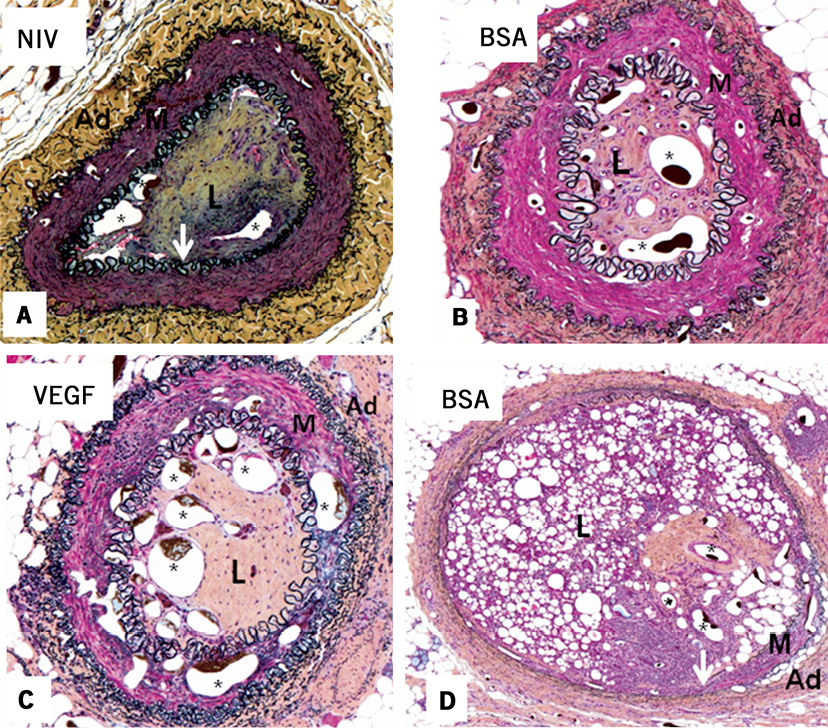
Figure 8. Movat pentachrome stained CTO arterial cross-sections. A) Non-interventional control CTO. B) BSA-treated microspheres. C) VEGF164-treated microspheres. A robust neovascularisation response is evident in the intima of the VEGF164 microsphere-treated artery, with less neovascularisation present in panels A and B. D) Residual microspheres (identified as small circular structures without endothelial cells and not containing microfil) at three weeks after BSA microsphere treatment of CTO. White arrows indicate internal elastic lamina.
IN VIVO GUIDEWIRE CROSSING STUDY
In vivo guidewire crossing of the CTO was attempted in 10 VEGF164-treated CTO arteries. Complete crossing was observed in six cases; in eight cases the guidewire successfully crossed the proximal cap and was advanced to the distal cap. The soft tip Choice PT extra-support guidewire successfully crossed the proximal fibrous cap in eight cases and crossed the entire CTO in two cases. A stiffer tip Confianza guidewire was used in six cases to cross the distal CTO, and was successful in four of these cases, where the soft tip guidewire could not cross. The mean time required for crossing was 19 minutes (range 4-35 minutes). The two cases of failed guidewire crossing occurred in blunt occlusions, where it was not possible to find the opening into the CTO. In five of nine CTO examined histologically, microsphere remnants were still evident at three weeks after injection.
Discussion
CTO are a common angiographic finding in patients presenting with symptomatic coronary artery disease, occurring in approximately 13-33% of angiograms13,14. However, <10% of CTO are treated by percutaneous coronary interventions, primarily due to low success rates in guidewire crossing. Our study is the first to administer a potent pro-angiogenic agent in order to facilitate guidewire crossing outcomes in CTO. A previous study from our group on CTO maturation indicated an early period of intense neovascularisation following experimental occlusion creation, with prominent regression of intraluminal microvessels at later time points3. This microvascular network has been recognised as an important predictor of CTO crossing by guidewires4, either as a pathway for the guidewires or by altering the mechanical and structural properties of a CTO. Thus, the rationale of this study was to determine whether microvascular formation within the CTO could be augmented in older CTO, and whether this could facilitate guidewire crossing. The study was performed in a well-validated CTO model3,11,12, which has been characterised at this time point as a mature, avascular CTO containing extensively fibrotic extracellular matrix (particularly in the proximal cap), which is extremely challenging to cross with conventional guidewires11.
Since VEGF plays a leading role in angiogenesis cascades15,16, VEGF164 seemed a reasonable initial approach. Previous studies have shown an angiogenic response at low doses of VEGF in the cardiovascular system17,18. Therapeutic angiogenesis with VEGF (either by recombinant protein or gene transduction) was shown to augment perfusion19,20, and has been used in various clinical settings of acute and chronic vascular insufficiencies resulting in regional tissue ischaemia21. Local delivery of VEGF directly into the CTO was preferred rather than a systemic delivery approach, since previous studies have shown that the half-life of the VEGF in the blood circulation is only a few minutes22, and only a small proportion of a systemically-administered VEGF would be accessible to the CTO. We also utilised a protein form of VEGF for these studies to ensure we knew the precise dose delivered to the CTO. In order to increase the residency time of the angiogenic agent at the CTO site, and hence to augment the treatment outcome, we utilised a polylactide-co-glycolide biodegradable sustained drug delivery system suitable for intravascular delivery.
The mass of VEGF164 released from the microspheres at the 24-hour time point in the in vitro study was 22 ng, a mass which has previously been shown to induce endothelial cell growth23. The current study showed a sustained, exponential release of VEGF164 from PLGA microspheres, with peak levels realised over 24-48 hours and accumulated plateau dose reached by 96 hours. The VEGF164 microspheres used in our studies were co-encapsulated with BSA to create a more porous internal architecture to ensure that VEGF was able to diffuse in a clinically relevant time frame. The initial burst release has been suggested to be due to VEGF164 located on the sphere surfaces, with subsequent release resulting from degradation of the polymer matrix24. Following the initial burst, VEGF164 release is likely controlled by diffusion and polymer degradation. Although the initial burst accounts for 10% of the total protein released, the release rate decreases substantially over the following four days. Silva et al showed that a high early concentration of VEGF followed by lower concentrations is capable of inducing more endothelial cell sprouting in vitro than a constant VEGF concentration, which supports our results23.
The angiogenic response within the CTO was confirmed by three different and complementary assessments of neovascularisation. MRI-determined blood volumes allowed a comparison between interventions from the pre-intervention state to three weeks later. Three-dimensional volume rendering using µCT imaging reconstructed the actual architecture of microvascular tree and provided a quantitative measurement of three-dimensional vascular volume at the final study time point. The µCT images also clearly differentiate between microvascular network within the external elastic lamina12, which was our interest in this study, and the more peripheral vascular structures outside the vessel wall. The histologic analysis of vessel cross-sectional area confirmed the neovascular response in the most proximal location of the CTO compared to BSA-treated and non-interventional CTO. The consistency of the results by all three techniques is also important since each imaging modality assesses different aspects of neovascularisation. A potential limitation of MR angiography is that the vascular volume may be overestimated since it may also include vessels outside the external elastic lamina. Conversely, µCT measurements of blood volume may be low due to incomplete filling of the vessels, either related to technical difficulties with the injection, limited size of the vessels or incomplete visualisation in the data segmentation due to low sensitivity. Histologic measurements may also be reduced by missing microvessels that have collapsed or by sampling error in the sections that are selected for analysis. Despite these potential technical differences in assessment of neovascularisation, it is reassuring that all three modalities consistently showed significant increases in neovascularisation in the VEGF164-treated arteries compared to the other two groups.
To assess the potential procedural significance of the increased microvessel formation within the CTO, we performed a pilot study to assess guidewire crossing. The overall crossing rate was 60%, which is comparable to previously published results of collagenase in this same model11, where placebo success rates are approximately 35%. In the proximal region of the CTO that underwent the neovascularisation response, we had 80% success rates using soft tip wires. This region represents the proximal fibrous cap and the proximal body of the CTO, which are typically barriers to CTO crossing. The two failures in crossing were specific cases where there was a flush occlusion and the actual entry site into the CTO could not be identified. However, a much larger trial involving placebo groups is required to verify these findings.
CLINICAL SIGNIFICANCE OF EXPERIMENTAL FINDINGS
Our data show that a biologic intervention can alter the tissue composition of CTO and restore levels of neovascularisation similar to an earlier stage of maturation. Previous clinical studies have indicated high success rates at these early stages of CTO development. The pilot studies of guidewire crossing confirmed high rates of success in crossing the proximal fibrous cap barrier and body of the CTO in VEGF164-treated CTOs. The vascular analysis showed that the VEGF164 effect on microvessel formation was predominantly restricted to the proximal part of the CTO. This is a limitation of the current approach and probably explains the requirement for stiffer guidewires to cross the more distal regions of the CTO. Further efforts to increase the distance of the neovascularisation across the entire length of the CTO should further enhance the effectiveness of the therapy. However, a much larger trial involving placebo groups is required to verify these findings. Potential modifications of this novel approach include combination growth factors (PlGF, angiopoietins, interleukin-8, hypoxia-inducible factor -1) or injection of pro-angiogenic precursor cells, such as bone marrow stem cells or endothelial progenitor cells, in addition to efforts to deliver this therapy more distally into the CTO.
Limitations of the study
The rabbit model we have utilised differs from the human coronary CTO due to the normocholesterolaemic diet and absence of background atherosclerotic lesions. The neovascularisation response we have observed in these adolescent rabbits may not be duplicated in more elderly patients. The delivery technique involving microspheres is outside of current clinical use and clinicians could be reluctant to use it.
Conclusions
Local delivery of VEGF164 protein into chronic total occlusions increases microvessel formation in the proximal CTO, and appears to facilitate guidewire crossing across the proximal fibrous cap and the body of the CTO.
Funding
Supported by the Canadian Institute of Health Research (grant number MOP -53325; grant number CTP 82943).
Conflict of interest statement
B. H. Strauss has applied for a patent for the use of angiogenic therapy in chronic total occlusions. The other authors have no conflicts of interest to declare.

