Abstract
Aims: To investigate the effectiveness of combining collagenase and ultrasound-stimulated microbubble (USMB) treatments in reducing the mechanical force required for crossing a guidewire through CTOs.
Methods and results: Experiments were conducted on ex vivo specimens of a rabbit femoral artery CTO model (n=45 total samples). Four primary groups were employed: control (n=6), collagenase only (n=15), USMB only (1 MHz frequency) (n=5), and collagenase+USMB (n=19). In one set of experiments the force required to puncture through CTO samples was measured and it was found that the puncture force was 2.31-fold lower for the combined treatment group relative to the comparable collagenase-only group (p<0.05). In a second set of experiments, the total protein and hydroxyproline content of the supernatant solution adjacent to the CTO was analysed. Significantly higher hydroxyproline levels were measured in collagenase+USMB treated CTOs (0.065 g/mL) compared to collagenase (0.030 g/mL), USMB (0.003 g/mL) and control (0.004 g/mL) (p<0.05), indicating that the combined treatment augmented collagenase degradation.
Conclusions: Ultrasound-stimulated microbubbles improved the effectiveness of collagenase in reducing the force required to cross experimental CTOs. This new approach may have the potential to reduce treatment times and improve the success rates of emerging collagenase-based treatments of CTO.
Introduction
Chronic total occlusion (CTO) is defined angiographically as an artery with Thrombolysis In Myocardial Infarction (TIMI) 0/1 flow that has been occluded for at least 12 weeks duration1. As these lesions age, their composition changes from a relatively soft thrombotic material to a hard, predominantly collagen-rich occlusion2. The presence of collagen is correlated to the age of the occlusion, which is a predictor of successful guidewire crossing3. The entrance into the CTO, known as the proximal fibrous cap (PFC), is characterised particularly by densely packed collagen2. The PFC appears to be a significant impediment to advancing a guidewire into the body of the CTO.
Previous preclinical studies have shown that locally delivered collagenase facilitates guidewire crossing in experimental CTOs4,5. The first-in-man Collagenase Total Occlusion-1 (CTO-1) clinical trial recently reported a 75% success rate in guidewire crossing after local injection of collagenase in coronary CTOs that had previously undergone failed attempts at percutaneous revascularisation6. However, a drawback of this therapy is the apparent necessity of an overnight (~18 hours) incubation period for collagenase effect prior to the guidewire crossing attempt4. Refinement of the procedure to shorten the waiting period to several hours to enable same-day guidewire crossing would be desirable. We hypothesised that ultrasound-stimulated microbubbles (USMB) may have potential to accelerate the effects of collagenase on CTOs. Indeed, there have been numerous previous studies investigating the use of ultrasound to potentiate the degradation of flow-occluding thrombus, one of the more prominent of which has been to use USMB in conjunction with the thrombolytic enzymes urokinase or tPa7-9. Microbubbles are systemically injected encapsulated bubbles currently in clinical use as diagnostic ultrasound contrast agents. However, when they are exposed to sufficiently high pressure amplitudes in the context of therapeutic ultrasound, they can vibrate violently, collapse and induce a range of bioeffects in target tissues. In this study, we investigated the feasibility of using adjuvant USMB treatments to accelerate the effects of collagenase therapy in an experimental CTO model.
Methods
This study was performed using an experimental model of chronic total occlusion with experiments carried out on ex vivo samples. Samples were excised from animals after sacrifice and treated under controlled conditions. Samples were studied under mechanical and biochemical testing. Mechanical tests were performed to measure the force required to puncture the proximal fibrous cap of the CTO. Biochemical analysis was performed to quantify the level to which collagen in the proximal cap was degraded during treatment.
CTO MODEL
All animal care and handling was performed in accordance with the guidelines specified by the Canadian Council on Animal Care (CCAC) for the care and use of laboratory animals and was approved by the Institutional Animal Care and Use Committee. Chronic total occlusions were created in rabbit femoral arteries using a thrombin injection model, as previously described10. Briefly, both femoral arteries of New Zealand White rabbits aged approximately 10-14 weeks were surgically isolated and clamped at two points approximately 1 cm apart to inhibit blood flow between the clamps. Thrombin was injected directly into the vessel in the isolated region to promote thrombin formation. The clamps were removed after one hour. The animal was recovered, and a CTO was allowed to develop for a total of 12 weeks following thrombin injection. This technique produces CTOs that closely mimic human coronary CTOs, including the presence of microchannels11, collagen accumulation, and well-developed proximal fibrous caps2. Twelve-week-old CTOs were used in the study since this time point has previously been characterised for significant collagen accumulation2, by marked increases in puncture force compared to earlier time periods12 and by low guidewire crossing success rates in both human coronary CTOs and the rabbit femoral artery CTO model1,4,13.
TISSUE HARVESTING
Prior to animal sacrifice and removal of a CTO sample, a stent was deployed 5-10 mm proximal to each CTO lesion. This facilitated identification of the proximal entrance into the CTO and also acted as a brace to support the extracted CTO vessels in the treatment and testing apparatuses12. Vessels containing the CTOs were harvested using gross dissection techniques. Segments of approximately 3-5 cm in length distal to the stent position were removed from the animal. Vessels were finely dissected under microscopic guidance to remove as much peripheral tissue as possible while avoiding vascular damage. Dissected samples were placed in 0.9% saline solution and treated as described below immediately after sacrifice.
TREATMENT GROUPS
Arterial samples were separated into four primary groups (Figure 1, Table 1): control (Group I) (n=6); collagenase-only (Group II) (n=15); ultrasound-mediated microbubbles (Group III) (n=5); and combined collagenase and ultrasound-mediated microbubbles (Group IV) (n=19). The collagenase-only group was divided into two subgroups: standard treatment time (2.5 hrs) (Group IIa) (n=10) and extended treatment time (5 hrs) (Group IIb) (n=5). The combined collagenase/ultrasound group was also separated into two subgroups: sequential treatment (Group IVa) (n=11) and concurrent treatment (Group IVb) (n=8). The study groups were designed based on the resource estimation method suggested by Mead14 for exploratory experiments for the four primary groups (Groups I, IIa, III, IVa). Using sample sizes of a minimum of five per group results in a total of 15 degrees of freedom, which meets the Mead criterion for maintaining degrees of freedom between 10 and 20 (19[number of samples]-3[number of groups]-1[blocking component]). After initial results were obtained, additional experiments to a) assess the effects of extending the collagenase treatment time and b) to determine whether or not there was a difference between performing USMB treatments concurrently with collagenase vs. USMB treatments sequentially with collagenase were undertaken. Ultimately, the number of degrees of freedom in the experimental groups (22) operate near the 20 degree upper bound of the desired number of degrees of freedom under Mead, using a worst case assumption of five samples per group, as is the case in the experiment (29[number of samples]-5[number of groups]-2[blocking groups]).
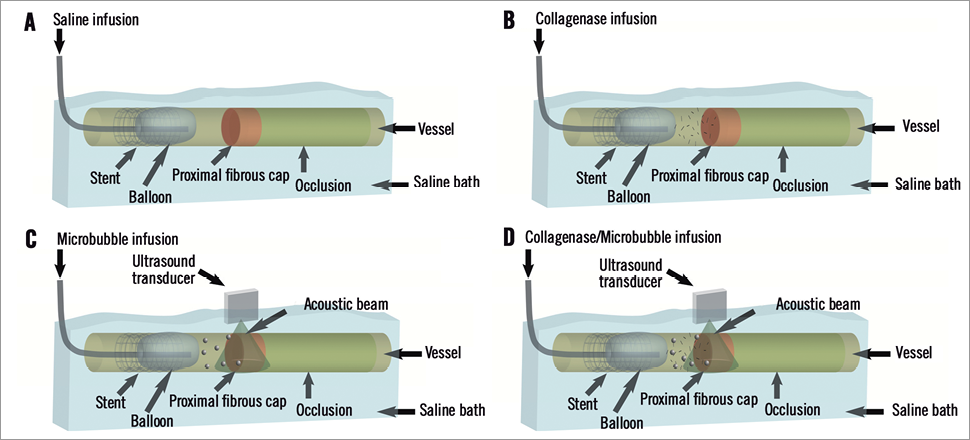
Figure 1. Schematic illustration for treatment groups. Panel A shows control group using only a saline infusion, while panel B shows the collagenase-only group. Panels C & D show groups using ultrasound to disrupt microbubbles. Panel C shows the ultrasound-only group, and panel D shows the “both treatments” group.
STANDARD TREATMENT
Each arterial sample was placed in a custom-built treatment reservoir in 10 mL of solution, with the contents of the solution depending upon treatment group. Prior to treatment, an over-the-wire (OTW) angioplasty balloon catheter (Long Cobra™; SciMed/Boston Scientific, Fremont, CA, USA) was advanced into the stent and inflated with the balloon tip ~5-10 mm from the CTO entrance to localise the proximal region. Depending on the treatment group, three infusions of 500 µL of either saline (Groups I, IIa, and IIb) or microbubble solution (Groups III, IVa, and IVb) were delivered via the central wire port of the OTW balloon for acoustic stimulation. Infusions were made with a two-minute interval time with a constant inflow of solution. Next, one infusion of 500 µL of either saline (Groups I and III) or collagenase (Groups IIa, IIb, IVa, and IVb) was delivered to the CTO site. The balloon was left inflated for 30 minutes. The sample was incubated at 37°C for an additional two hours, resulting in a total treatment time of 2.5 hours. 500 µL of the solution supernatant was withdrawn from the space proximal to the proximal cap and frozen at –20°C for supernatant protein analysis (see later section for details). The CTO sample was then taken for biomechanical puncture force testing (see below), which was performed immediately after incubation.
EXTENDED TREATMENT DURATION
In our previous in vivo collagenase study4, collagenase-only therapy was found to be ineffective at very short waiting periods (~1 hr). Therefore, to assess the time dependency and potential interactions between ultrasound and collagenase, we studied two treatment times: (1) standard treatment duration (2.5 hours, including 30 minutes of balloon inflation) (Group IIa) and (2) an extended treatment duration (5 hours, including one hour of balloon inflation) (Group IIb).
TREATMENT ORDER
The order in which ultrasound treatments are conducted may potentially have a significant influence on the efficacy of USMB therapy. Therefore, two subgroups of USMB and collagenase treatments were assessed: (1) USMB treatments conducted prior to collagenase therapy (Group IVa), and (2) microbubbles and collagenase injected together, with ultrasound exposures of the microbubbles occurring in the presence of collagenase (Group IVb).
ACOUSTIC STIMULATION PROTOCOL
The treatment reservoirs were designed to include acoustically transparent windows to allow ultrasound energy in and out of the reservoirs without significant reverberations so as to avoid standing waves (Figure 2). Ultrasound treatments were conducted with a 1 MHz centre frequency spherically focused transducer, with an aperture size of 3.8 cm and an F-number of 2 (Valpey-Fisher). The peak negative pressure was measured at 1.7 MPa in hydrophone tests (Sonora Medical Systems Inc., Model #804). For a given microbubble injection, the sample was subjected to an ultrasound exposure sequence consisting of a series of 25 ultrasound bursts separated by 5 s. Each burst comprised 500 16 µs long pulses, separated by 1 ms. The relatively low duty cycle (fractional “on-time”= 0.0016) was employed to avoid macroscopic thermal effects, the short individual pulses (16 µs) avoided the potential for standing waves, and the 5 s inter-burst timing permitted microbubbles to flow into the acoustic field. The nature of the sequence (pressure level and pulse types) ensured that microbubbles within the beam were disrupted following each individual burst (data not shown). The pulse sequence used is shown in Figure 3.
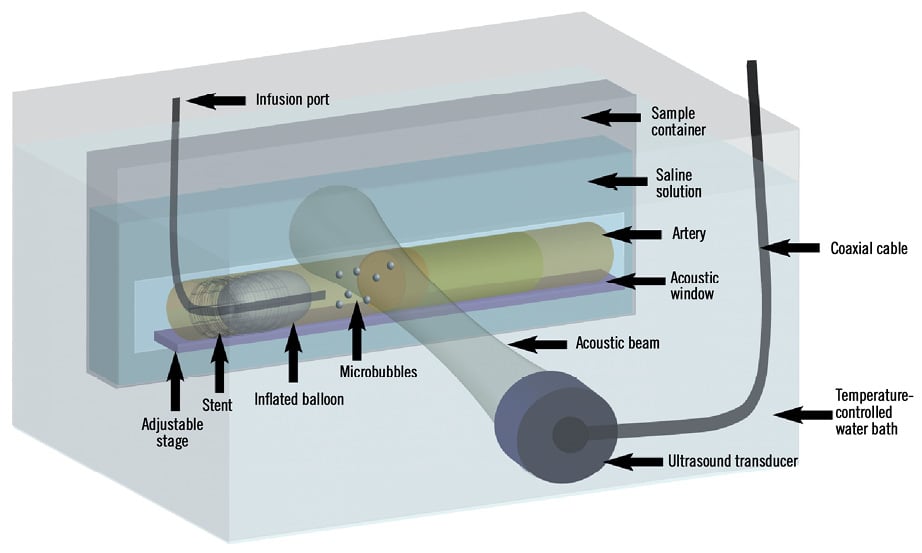
Figure 2. Ex vivo acoustic set-up. The vessel was secured into the sample container and placed onto the adjustable stage so that the proximal fibrous cap was located at the focus of the ultrasound transducer. A balloon catheter was advanced into the stent and the balloon was inflated to localise the proximal end of the lesion. Microbubbles were injected via the wire port of the catheter and disrupted in the acoustic beam.
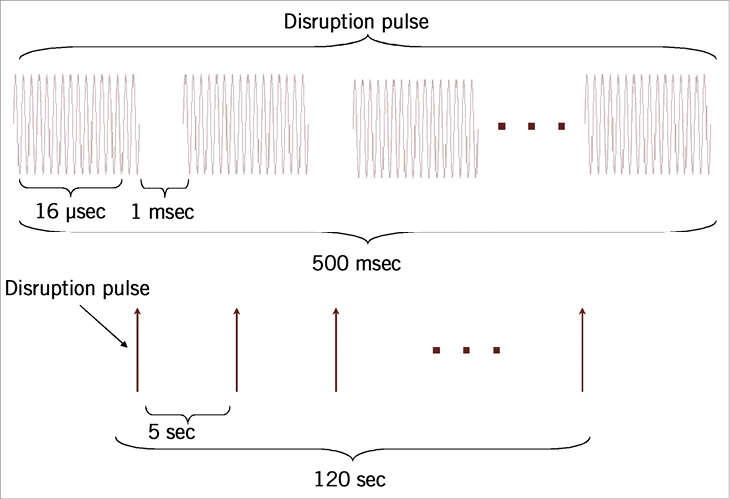
Figure 3. Pulse sequence for acoustic treatments. A 500 ms disruption pulse, consisting of 500 pulses of acoustic energy (16 cycles at 1 MHz), separated by 1 ms was used to disrupt the bubbles in the acoustic field. These pulses were sent every 5 s in order to allow fresh bubbles to flow into the field after a disruption sequence.
The microbubble agent Definity™ (Luminity™ in Europe) (Bristol-Meyers Squibb, New York, NY, USA) was employed, which is currently approved for clinical diagnostic imaging purposes. This agent has been used in preclinical studies to demonstrate improved thrombolysis in a rabbit model15. It is composed of phospholipid encapsulated perflutren bubbles having a wide range of diameters, with approximately half being between 1-5 µm in diameter, and the rest below 1 µm16. After activating agent vials according to the manufacturer’s instructions, the microbubbles were diluted to 10:1 by volume in phosphate-buffered saline (PBS).
Samples in the USMB-only (Group III) and combined (USMB+ collagenase) treatment (Group IV) groups were also exposed to three ultrasound treatments, each lasting 120 s.
COLLAGENASE
Type VII-S bacterial collagenase derived from Clostridium histolyticum (Sigma, c2399) was used at a concentration of 1,000 collagen digestion units (CDU) in 300 uL of PBS. Unlike human or other mammalian collagenase, collagenase from Clostridium histolyticum cleaves collagen at numerous sites, leaving several very small peptides17. The formulation used in this study was very similar to that used in the CTO-1 trial6.
SUPERNATANT PROTEIN ASSAYS
The supernatant solution extracted at the end of treatment was analysed to infer the level of collagen degradation in each group. Two different assays were performed to examine the degradation products released at the site of the occlusion. First, a Biorad® DC protein assay was performed to estimate the total quantity of protein products released. This is a colourimetric assay based on a method developed by Lowry et al18 and modified to be detergent compatible. Briefly, 50 µL of supernatant solution was placed in a tube, mixed with 500 µL of Biorad® reagent A, and 2,000 µL of Biorad® reagent B. The sample was vortexed and left for 15 minutes to allow the colour to develop. Bovine serum albumin (BSA) standards were prepared in concentrations ranging from 0.2 to 2.5 g/L. These standards were exposed to the same reagents as the samples, as described above. Absorbance was measured at 750 nm using a KC4 plate reader (BioTek®, Winooski, VT, USA). This assay was used to determine if any increased collagen degradation was due to a non-specific increase in all proteins in solution or due to a specific increase in collagen degradation alone.
Second, a hydroxyproline assay was carried out to determine the quantity of products in the supernatant solution released from collagen degradation. Collagen and elastin are the only two extracellular matrix proteins containing significant amounts of hydroxyproline19. However, since collagen is by far the more predominant matrix protein, determination of total hydroxyproline is an appropriate measurement of collagen20. The protocol used for this colourimetric assay was a modified version of the techniques described by Reddy et al21 and those described by Edwards et al22. For this assay, 50 µL of the supernatant solution was extracted and placed into a 2 mL screw-capped tube with a rubber O-ring in the cap (UltiDent Scientific, St. Laurent, QC, Canada) containing 100 µL of 6N HCl. Similarly, standards from pure hydroxyproline (Sigma-Aldrich®, St. Louis, MO, USA) for concentrations up to 120 µg/mL were made up and placed in screw-capped tubes containing HCl. The tubes were heated at 120°C for four hours to hydrolyse the collagen in the solution. After heating, the tubes were placed in a speed-vacuum (Savant Instruments Inc., Farmingdale, NY, USA) and dried for four hours. Once dried, 450 uL of chloramine-T solution (made up from 0.127 g chloramine-T [Sigma], 2 mL of 50% n-propanol, diluted to 10 mL with acetate buffer) was added to each tube and allowed to react for 25 min at room temperature. Acetate buffer was made in batches from 120 g sodium acetate, 46 g citric acid, 12 mL acetic acid, 34 g NaOH, and brought to 1 L by dilution with autoclaved water. Then, 500 µL of Ehrlich’s reagent (made up from 1.5 g p-dimethyl-amino-benzaldehyde [Sigma], 6 mL n-propanol, 2.6 mL perchloric acid, diluted to 10 mL with autoclaved water) was added and allowed to incubate at 65°C for 20 min. 200 µL of each reacted sample was pipetted into a 96 well plate and taken to a plate reader to have absorbance values at 550 nm measured. Samples were compared to standards to determine hydroxyproline concentrations, which are reflective of total collagen degraded from the CTOs and released into the solution. All supernatant samples were performed in triplicate, while standards were performed in duplicate.
PUNCTURE FORCE TEST
We recently developed a method to measure directly the puncture force required to penetrate the PFC12. Similar techniques have been used for rabbit ear artery puncture, as well as various other applications23,24. A custom-built system for measuring PFC puncture force was used (Figure 4). The stent was clamped with enough force to avoid slipping but not enough to collapse the stent, and the vessel was laid onto the mounting plate. The muscle tissue peripheral to the vessel of interest was gently stretched and secured with a pin at the distal end to maintain vessel length. The puncture probe was carefully placed at the PFC, and the mounting plate was secured into a saline bath containing 0.9% NaCl heated to 37°C. The specimen was positioned such that the puncture probe was vertically aligned with the axial centre of the stent. The load cell was zeroed. The microtester (TestResources 800LE; TestResources Inc., Shakopee, MN, USA), which is electronically controllable in the vertical axis, was slowly lowered at a rate of 0.05 mm/sec. As the probe moved, a force was applied to the probe, which was measured by the load cell. The force-displacement (f-d) curve was recorded. The f-d curve was analysed for puncture. A puncture of the PFC is signalled by a steady rise on the f-d curve followed by a rapid drop-off. A sample force-displacement curve from a control (Group I) sample is shown in Figure 5.
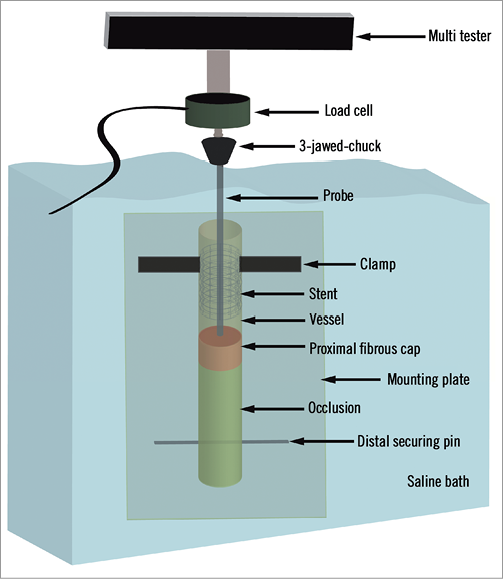
Figure 4. Biomechanical testing set-up. The stent is clamped with the vessel lying on the mounting plate. The distal end of the vessel is held taut with the distal securing pin. The puncture probe is then lowered into the occlusion. The mounting plate is mounted into the tank which is filled with temperature-controlled saline. The probe is then connected to the load cell assembly via the 3-jawed-chuck. The assembly is slowly lowered into the vessel until the proximal fibrous cap is punctured.
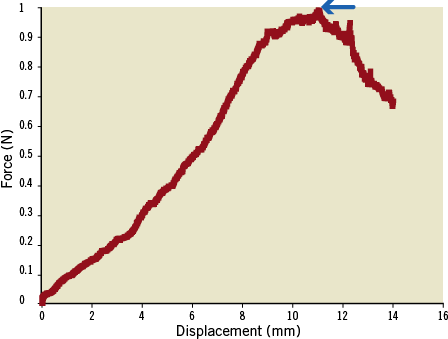
Figure 5. Force displacement curve for a control sample CTO. Blue arrow shows puncture point at peak of curve. In the case shown, the puncture force was measured to be approximately 1N.
STATISTICAL ANALYSIS
Data are reported as mean±standard error of the mean (SEM). Groups were compared together using ANOVA analysis. If significant difference was present with ANOVA analysis, groups were compared pairwise using two-sample t-tests, making no assumption of equal variance between groups. A p-value of <0.05 was considered significant.
Results
STANDARD TREATMENT LENGTH
SUPERNATANT PROTEIN ASSAYS
There were no significant differences in total protein in the supernatant among the four primary groups (Figure 6). There were, however, significant differences in hydroxyproline (collagen) content among the groups (Figure 7). In both the control and ultrasound-only (Groups I & III) cases, minimal hydroxyproline levels were detected in the supernatant (0.004 and 0.003 g/L, respectively). The collagenase-only treatment (Group IIa) showed a marked increase in hydroxyproline concentration, 7.50-fold greater than the control group (Group I) (0.030 vs. 0.004 g/L, respectively, p<0.01). The addition of the ultrasound treatment to the collagenase therapy (Group IV) resulted in a significant 2.17-fold increase in hydroxyproline level compared to the collagenase-only (Group IIa) (0.065 vs. 0.030 g/L, p<0.05).
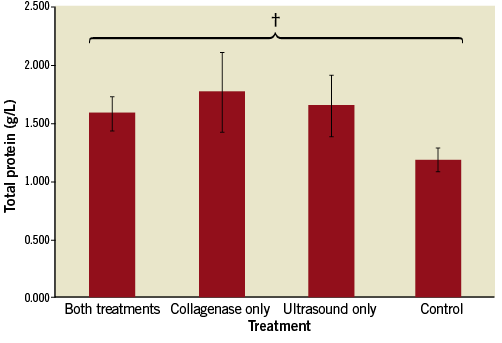
Figure 6. Total protein released into medium for standard treatment duration. There was no statistically significant difference in the total protein released in any of the groups. † p=NS (not significant).
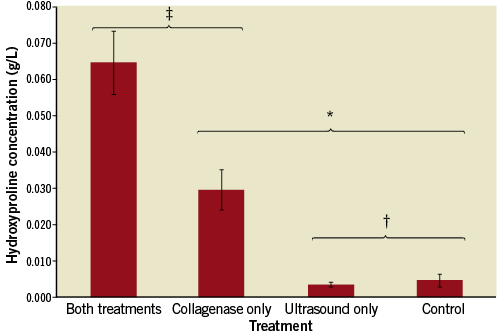
Figure 7. Hydroxyproline released into medium for standard treatment duration. Both the combined treatment and collagenase-only groups showed significant increases in hydroxyproline release compared to ultrasound-only and control. Hydroxyproline in the combined treatment group was significantly higher than in the collagenase-only group. * p<0.01; ‡ p<0.05; † p=NS.
PUNCTURE FORCE TEST
The results for the puncture test are shown in Figure 8. The peak force required to puncture a CTO was significantly reduced in the combined collagenase-ultrasound treatment (Group IV) compared to all three other groups (p<0.05). Specifically, it was reduced by a factor of 2.31 and 2.05 compared to the collagenase-only (Group IIa) and control (Group I) groups, respectively, and a factor of 3.21 compared to the USMB-only group. There were no statistically significant differences between control (Group I), collagenase-only (Group IIa), and ultrasound+microbubbles (Group III).
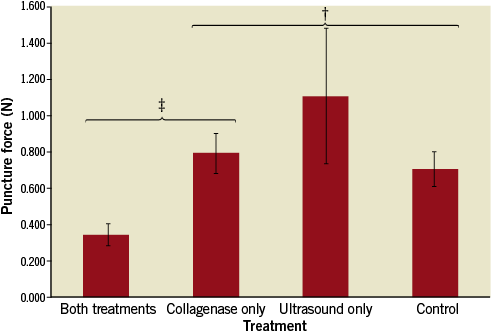
Figure 8. Puncture force test result. There was a significant reduction in puncture force in the combined treatment compared to the collagenase-only group. ‡ p<0.05; † p=NS.
EXTENDED TREATMENT DURATION
Extending the collagenase-only treatment time from 2.5 hours (Group IIa) to 5 hours (Group IIb) had significant effects on both the hydroxyproline assay and the puncture force test (Figure 9). The extended treatment arm showed a 4.05-fold increase in hydroxyproline (0.135 vs. 0.030 g/L, p<0.01) and a 1.82-fold reduction in the puncture force (0.44 N vs. 0.80 N, p<0.05), compared to the standard duration arm.
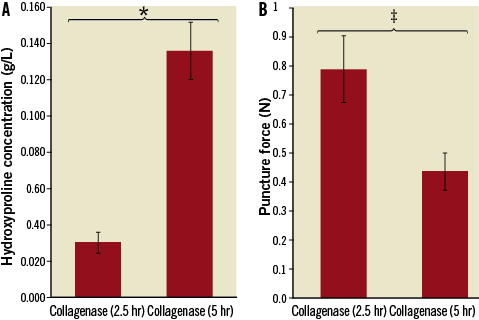
Figure 9. Extended treatment duration results. The extended treatment group showed a significant increase in hydroxyproline release and a significant reduction in the required puncture force. *p<0.01; ‡p<0.05.
TREATMENT ORDER
The sequential (Group IVa) and concurrent treatment (Group IVb) groups showed similar hydroxyproline levels (0.065 vs. 0.062 g/L, p=NS) and puncture forces (0.300 N vs. 0.388 N, p=NS) (Figure 10).
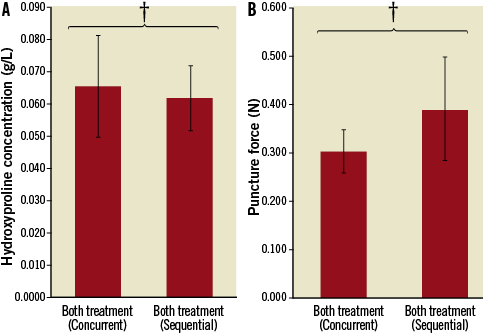
Figure 10. Modified acoustic set-up results. Hydroxyproline and puncture force assays are shown. In both cases, there were no differences between the two treatment configurations. † p=NS.
Discussion
The major finding of this study is that combined USMB and collagenase therapy increases the degradation of collagen and decreases the puncture force required to cross the proximal cap of a CTO compared to collagenase alone. Combining USMB with collagenase (Group IV) lowered the puncture force by a factor of 2.31 compared to the collagenase-only group (Group IIa), which suggests that a combined therapy is a promising adjunct to collagenase therapy. Within the collagenase-only group, the reduction in puncture force was evident in the five-hour treatment group (Group IIb) but not in the 2.5-hour group (Group IIa). This is also consistent with previously reported results that local delivery of collagenase followed by a short waiting period did not facilitate guidewire crossing4. These results may therefore have significant clinical relevance by shortening the waiting time after collagenase administration and potentially allowing a single-day procedure rather than the current requirement of a two-day procedure.
An additional important finding of this study is that there were no significant differences whether the collagenase was delivered after or concurrent to USMB therapy: in both cases the combined therapy significantly reduced puncture force levels relative to the collagenase-only case. This is a very useful result from a practical perspective, since the delivery of microbubbles through a balloon catheter while the balloon is inflated will subject them to high ambient pressure levels that will cause their collapse and likely render them ineffective for USMB therapy. Since the option to deliver the bubbles without balloon inflation is viable (i.e., Group IVa), this approach can therefore be employed in future in vivo and clinical studies.
While puncture force reductions are the most directly clinically relevant parameter assessed in this study, the supernatant protein assays provide important insights into the effects of treatment on the collagen-rich PFC. The treatments that used collagenase were shown to be selective for collagen since there were no significant differences among the total protein levels in any of the treatment groups. This suggests that there is a low background level of protein release related to the testing conditions, which is not disturbed by either the collagenase or USMB treatments. As hydroxyproline is a commonly used biomarker for the presence of collagen –either intact or degraded– any increase in the hydroxyproline content for a given treatment can be attributed to an increase in collagen degradation, and not just to an increase in non-specific protein. As expected, the control group (Group I) showed negligible amounts of collagen products in the supernatant since no collagen degradation agent was present. Similarly, the USMB-only group (Group III) showed minimal hydroxyproline levels, as there was no enzyme present to degrade the collagenous PFC. The collagenase-only group showed a 7.50-fold increase in the amount of collagen released into the supernatant, indicating the presence of large amounts of collagen in the proximal fibrous cap as previously shown by our group. There was a further 2.17-fold increase in the hydroxyproline levels in the combined collagenase/ultrasound group (Group IV). The effect of ultrasound appears to be synergistic with collagenase since the USMB-only group (Group III) showed minimal collagen release, similar to the controls.
Although the hydroxyproline content in the supernatant was higher in the extended collagenase treatment (Group IIb) compared to the combined therapy used in Group IV (0.135 vs. 0.063 g/L, respectively), this was not accompanied by a corresponding reduction in the puncture force required to cross the lesions (0.440 N vs. 0.345 N, respectively, p=NS). The very low puncture force demonstrated in this study under the combined treatment groups (Groups IVa and IVb) provides further support for the use of USMB since a similar effect on puncture force was achieved with half of the collagenase exposure time and, consequently, there was less potential for degradative effects on deeper layers of the vessel wall.
This study has demonstrated clear enhancements of the therapeutic effects of collagenase by combining it with USMB treatments, but has not examined the potential mechanisms for this synergy. It is useful to note that, for the closest analogue to this treatment, USMB potentiated thrombolysis with thrombolytic enzymes, mechanisms of action remain largely a matter of speculation. In this context it has been hypothesised that oscillating microbubbles near thrombus boundaries cause increased penetration of enzymes or may cause local thermal elevations which act to increase lytic activity (enzymes administered concurrently). Interestingly, our results show that required puncture forces are reduced even when the USMB treatments occur prior to the introduction of collagenase, which indicates that improved penetration of collagenase due to the presence of oscillating microbubbles would not be a factor. These results would, however, be consistent with USMB treatments inducing a degree of damage to the PFC surface of the CTOs, which may subsequently improve collagenase penetration. Indeed, the exposure levels we have employed induce microbubble destruction, which can be associated with potentially violent microbubble oscillations. It is well known that strongly oscillating bubbles near boundaries can produce “microjets” directed at the boundary which can in turn potentially induce damage to the surfaces. The elucidation of the mechanisms of action is the subject of ongoing work.
It should also be noted that this study complements earlier studies showing that a collagenase therapy can improve guidewire crossing success rates by degrading the PFC. Thus, our data in this study further reinforce the mechanism by which collagenase works in CTOs: collagen is degraded from the PFC and lowers the puncture force required to cross the occlusion to levels typically associated with chronologically younger (and more easily crossed) occlusions.
STUDY LIMITATIONS
This study did not examine the effects of damage to the vascular wall –acute or chronic, either through microbubble disruption, collagen degradation, or a combination of the two. This was not achievable within the confines of an ex vivo study, as the physiologic response to any potential injury could not be modelled in the ex vivo setting. It is worth noting that a preferred approach to adapt the in vitro methodology to a clinical setting would be to use ultrasound stimulation with a catheter-based transducer that can preferentially direct energy in a forward direction towards the CTO. This should avoid stimulation of microbubbles adjacent to the normal vascular wall proximal to the CTO, along with potential unintended bioeffects. The development of this device would require additional validation for effectiveness and safety in preclinical models.
Moreover, since this is an ex vivo study, the vessel has been removed from its native environment. It is well understood that bridging collaterals often form new pathways across CTOs. The process of harvesting the vessel may sever some of the collaterals, providing a leakage path for infused solution, and reducing the resistance to flow injection through the OTW balloon.
Finally, the optimisation of either the collagenase dose or of the acoustic exposure parameter space was not performed. A single set of acoustic parameters that are likely achievable on catheter-based probes was used. However, there is considerable latitude to modify a number of acoustic parameters such as the pulsing schemes, pressure levels and frequencies, and these will be an area of focus in future work. This is similarly true of collagenase dose considerations.
CLINICAL RELEVANCE OF THE STUDY
Local delivery of collagenase directly into chronic total occlusions has been shown to result in high guidewire crossing rates in both experimental CTOs and in human CTOs treated following failed guidewire crossing attempts during the CTO-1 trial6. While these previous studies showed excellent promise, they require overnight incubation in order to soften CTOs. Refinements that may accelerate collagenase activity and shorten the waiting time prior to attempting to cross the CTO are desirable. The feasibility of the procedure in terms of patient and physician acceptance and cardiac catheterisation lab efficiency would be improved by the ability to perform both parts of the procedure (collagenase delivery and guidewire crossing) in a single day, rather than over two days as the current clinical protocol maintains. Moreover, it provides further support of the potential for therapeutic ultrasound in accelerating and enhancing enzyme therapies.
Conclusions
Local delivery of collagenase therapy into CTOs has previously been shown to assist guidewire crossing successfully and significantly when given adequate dwell time. This effect of collagenase therapy can be accelerated by using ultrasound-mediated microbubble disruption by increasing the total amount of collagen degraded in the lesion, resulting in a decreased puncture force of the proximal fibrous cap. Combined ultrasound/collagenase treatment appears to be a promising modification of collagenase therapy by shortening the required treatment period and potentially allowing for a single-day therapy for both treatment and revascularisation.
Funding
The study was funded by the Canadian Institute of Health Research (Grants # MOP -53325, CTP 82943). B. H. Strauss holds the Reichmann Chair in Cardiovascular Sciences at Sunnybrook Health Sciences Center. A. S. Thind is funded by the Department of Medical Biophysics, University of Toronto.
Conflict of interest statement
B. Strauss holds a patent on the use of collagenase for angioplasty in chronic total occlusions and is founder of Matrizyme Pharma, which is investigating collagenase for percutaneous interventions in chronic total occlusions. The other authors have no conflicts of interest to declare.

