Abstract
Aims: The aim of the study was to determine the safety, efficacy and feasibility of a new chronic total occlusion (CTO) device using optical coherence tomography (OCT) technology, the Ocelot catheter (Avinger, Inc., Redwood City, CA, USA), for crossing of SFA CTOs following guidewire failure.
Methods and results: Prospective, multicentre, market preference testing. Thirty-three patients with confirmed CTO (99-100% stenosis by visual estimate) of their superficial femoral artery (SFA) were treated between September 28, 2011, and December 9, 2011, at three European centres. Ocelot crossed 94% (31/33) of CTOs, allowing guidewire placement in the distal true lumen. All (100%) lesions were treated without any major adverse safety events. Procedural time and contrast dose were significantly reduced (p<0.0001) when compared with a similar, non-OCT-guided CTO crossing device (Wildcat catheter; Avinger, Inc.). Overall physician feedback on the catheter performance was positive with an 87% average rating of excellent or good across seven categories. Performance ratings of Ocelot’s OCT imaging guidance were consistently positive with an 86% average rating of excellent or good across five OCT categories.
Conclusions: The Ocelot catheter combines advanced CTO crossing technology with real-time OCT guidance. When compared with a similar non-OCT-guided catheter, crossing efficacy and safety profile improved. Total procedure time and contrast volumes were significantly reduced. The Ocelot is a safe, efficient and effective tool for crossing CTOs.
Introduction
It is estimated that peripheral artery disease (PAD) affects approximately 12-14% of the general population, and approaches 20% in patients 75 years of age or older1. Over 200,000 amputations are reported each year for patients with end-stage PAD in the United States and Europe2. As the population continues to live longer, the prevalence of this disease is only expected to grow. Without early diagnosis and risk factor modification, approximately 1-2% of these patients will develop critical limb ischaemia (CLI)3, defined as the presence of lower extremity rest pain, with or without gangrene or non-healing ulcers, caused by inadequate blood supply4. Without successful revascularisation, CLI patients have significantly increased morbidity with a 95% amputation rate at one year5.
The Inter-Society consensus (TASC) II guidelines traditionally recommend surgical intervention for severe PAD including peripheral arterial chronic total occlusions (CTO), denoted as type C and D lesions. Bypass surgery has traditionally been the “gold standard”, with a five-year limb salvage rate of nearly 80%6-9. Recent advances in endovascular therapy enable patients with CLI to be treated using minimally invasive techniques. With revascularisation reported as high as 97%, endovascular crossing options are becoming first-line crossing treatments for patients across a wide health spectrum. Importantly, percutaneous therapeutics are a safe alternative for non-viable surgical candidates as a result of their comorbidities, lack of suitable target vessels or poor venous conduits10.
Percutaneous crossing of infra-inguinal arterial CTOs is dependent on emerging guidewire and catheter technology. Guidewire therapy rests on the premise of intentional extraluminal (subintimal) crossing, as originally described by Bolia et al11. A recent large systematic review highlights one-year patency following subintimal angioplasty at 50%, with a limb salvage rate of 80-90%12. Alternatively, targeting intraluminal crossing may alleviate subintimal restenotic factors while enabling a wider spectrum of therapeutic interventions, including stenting and atherectomy. Adam et al13 demonstrated statistically equivalent one-year clinical success rates (56% vs. 50%) between surgery and angioplasty. By contrast, the surgical arm was associated with higher complications and costs.
In recent years, medical device companies have continued to develop and improve catheters for crossing CTOs in peripheral arteries. These include the CrosserTM (Bard Peripheral Vascular, Inc., Tempe, AZ, USA), TruePathTM (Boston Scientific Corp., Natick, MA, USA), the Frontrunner® XP (Cordis Corp., Bridgewater, NJ, USA), the CrossBossTM (BridgePoint, Plymouth, MN, USA) and the Wildcat catheter (Avinger, Inc., Redwood City, CA, USA).
While advancements in percutaneous CTO treatments are ongoing, a true paradigm shift is emerging with the advent of advanced intravascular imaging, including intravascular ultrasound (IVUS) and optical coherence tomography (OCT). These technologies provide another axis of image interpretation, an adjuvant to existing angiography, for successful CTO mapping and crossing.
IVUS, a well-established diagnostic and surveillance technique, has proven helpful in quantifying plaque morphology in coronary lesions14. Likewise, the optical properties of OCT enable a histologic grade differentiation of atheromatous plaque across the wide spectrum of intimal disease15,16. Rieber et al demonstrated the comparative advantages of OCT over IVUS in granular clarity when evaluating the lipid components of atheromas17. OCT consistently provided greater detail with respect to plaque composition, potentially leading to stronger indices for thin-cap fibroatheromas, the hallmark of vulnerable arterial disease leading to thrombosis, ischaemia and sudden cardiac death18,19.
OCT, a relatively new technology, has already made large impacts in clinical fields20 ranging from ophthalmology to gastroenterology21 and dermatology22. The discriminating optics range (10 μm) allows for an axial resolution in the order of 10-20 times greater than standard b-mode ultrasound imaging23. Initial OCT applications in the endovascular arena have focused on diagnostic capabilities. Using OCT, clinicians are able to map and quantify arterial plaque structures in vivo. Regar et al showed the early usefulness of OCT in identifying a variety of plaque structures including thin-cap fibroatheromas23. More recent studies have validated the superiority of OCT over IVUS for detection and characterisation of plaque structures including calcium, lipid pools and fibrin24. As a result of the growing OCT momentum in cardiovascular medicine, the International Working Group for Intravenous OCT (IWG-IVOCT) has recently released a consensus standardisation and validation in an effort to improve further both diagnoses and treatment of atherosclerosis25.
The Ocelot, the first-ever OCT-equipped CTO crossing catheter, utilises proprietary real-time OCT guidance and navigation for optimal intraluminal recanalisation. Ocelot’s optical image resolution is 10 μm and incorporates 1,024 A-lines per image. These capabilities enable differentiation between various healthy arterial structures, including media and adventitia (layered structures), and diseased arterial walls, including plaque (non-layered structures). Either saline or contrast may be used via the local flush port to clear the imaging fibre when blood refracts the OCT image. Minimal flush (<1 cc) is adequate to displace blood artefact. The static flow environment of a CTO minimises the need for large flush volumes or proximal balloon dilation. Ocelot’s working edge includes fixed spiral flutes with adjustable speed of rotation between 30, 45 and 60 rotations per minute and may be advantageous for crossing CTOs with live evaluation of luminal boundaries, improving recanalisation rates and ultimately limb salvage.
Methods
DEVICE DESCRIPTION
The Ocelot system consists of the Ocelot catheter and the Lightbox console (Avinger, Inc., Redwood City, CA, USA). The Ocelot system combines the use of advanced catheter design and real-time OCT for orientation and navigation of the catheter tip across chronic total occlusions.
OCELOT CATHETER
The Ocelot catheter is an over-the-wire device that is compatible with a 6 Fr sheath and a 0.014” guidewire, has a working length of 110 cm, and incorporates a 155 mm optical fibre. The catheter has a distal tip with spiral wedges (Figure 1) that can rotate clockwise and counterclockwise to facilitate a corkscrew-like action, allowing for advancement of the catheter across a CTO. Additionally, the distal tip is preshaped and contains radiopaque markers (Figure 2). The directional markers are used to orient the catheter during a CTO-crossing procedure. The middle directional marker (Figure 3) corresponds to the marker band located directly opposite the catheter’s distal tip deflection. As such, the operator guides CTO crossing by positioning the middle marker over healthy tissue (layered structures) prior to distal advancement of the catheter. This orientation permits the catheter tip to engage the diseased segments, allowing for truer luminal crossing and a better chance of re-entry. Real-time, constant OCT imaging allows for reorientation of the middle markers over layered structures for the duration of lesion crossing. This technique, referred to as OCT-assisted orientation, ensures that the deflected working edge of the Ocelot is pointing towards diseased tissue and away from healthy structures, as demarcated by the middle marker (Figure 4).
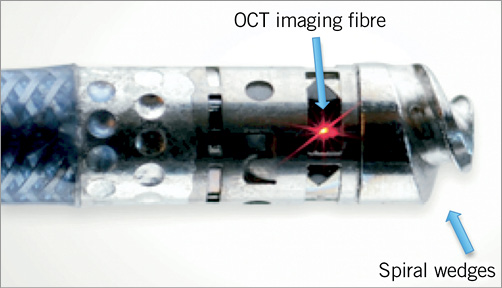
Figure 1. Distal tip of Ocelot catheter.
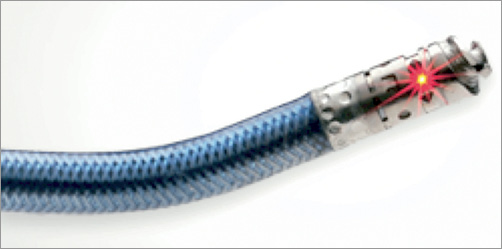
Figure 2. Pre-shaped distal tip deflection.
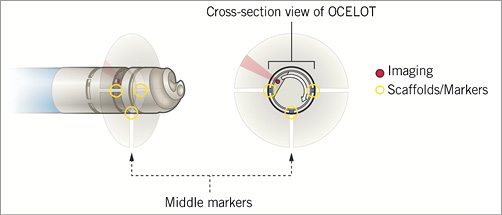
Figure 3. Directional markers on OCT system.
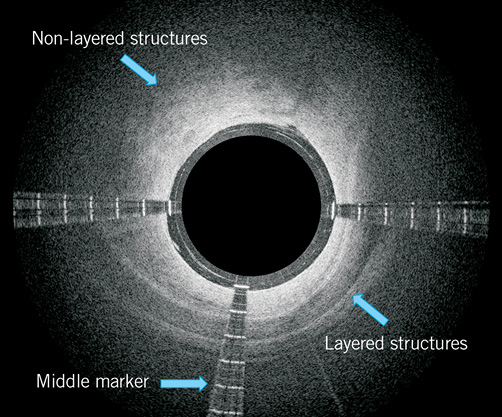
Figure 4. Ocelot OCT image with directional markers and layered structures.
The Ocelot catheter handle (Figure 5) controls rotational orientation of the outer catheter shaft independent of the rotational movement of the distal tip. Rotating the distal tip also allows for real-time cross-sectional OCT of the surrounding vasculature during the procedure. For improved image quality, small amounts of saline can be flushed through the distal tip using the luer located at the proximal end of the catheter handle assembly. After the catheter enters the distal reconstitution site, it is removed, leaving the wire in place for therapeutic interventional treatment.
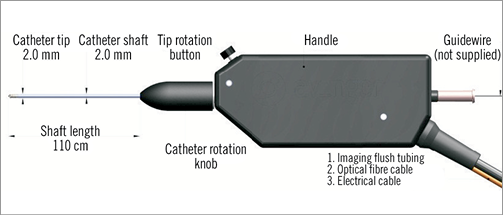
Figure 5. Ocelot catheter handle.
LIGHTBOX
The Ocelot catheter connects to the Lightbox console using an optical and electrical extension cable, which is used to provide an OCT image of the vasculature when coupled. The resulting image provides a qualitative intraluminal view of the vessel morphology as an adjunct to standard imaging techniques (e.g., fluoroscopy) (Figure 6). The Lightbox transmits light to the intraluminal environment through the optical fibre on the Ocelot catheter and receives and interprets the signal from the tissue using a PC-based processing system. The Lightbox processes the reflected light signal and displays a plot of tissue reflectivity versus depth, thereby creating an image of the surrounding vasculature. The Lightbox is supplied with two monitors, one monitor for physician viewing and the other used by the assisting Lightbox operator.
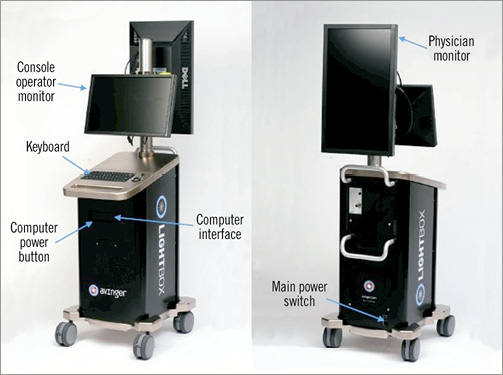
Figure 6. Lightbox console (front and rear view).
OCT images captured by the Lightbox are presented in two modes on the physician monitor: sector view and waterfall view. The sector view provides a cross-sectional intraluminal view during rotation of the distal catheter tip. The reflected OCT image is correlated to the distal tip’s deflection angle using the middle marker, while the directional markers are fixed with respect to distal tip rotation. The resulting OCT image can then be used to help the physician orient the distal tip and navigate within the vessel as an adjunct to standard visualisation tools (e.g., fluoroscopy).
The Ocelot System (Avinger, Inc.) received CE mark approval in September 2011.
DATA COLLECTION AND DEFINITIONS
After informed consent, patients with CTO of their superficial femoral artery (SFA) were treated in the market preference testing (MPT) study between September 28, 2011, and December 9, 2011, at three European centres. Ocelot catheter and OCT image performance data were based on direct physician feedback during each case and were rated on a Likert scale from 1-5 where: 1=unacceptable; 2=poor; 3=acceptable; 4=good; 5=excellent. Ocelot catheter performance feedback included: catheter introduction into sheath, device trackability, device pushability, tip deflection, ability to stay in true lumen, visibility of radiopaque markers, visibility of catheter tip under fluoroscopy. OCT image quality feedback included: visualisation of vessel wall layered structures, visualisation of vessel wall non-layered structures, visualisation of side branches, ability of saline flush to displace blood adequately for visualisation, ability to orient the device using the middle marker.
Definitions for key safety and efficacy measures were based on the CONNECT study26. The key safety measure was a composite endpoint capturing procedural major adverse events (MAE), clinically significant perforations requiring treatment, embolisations resulting in distal ischaemia and/or requiring rescue intervention, and grade C or greater dissections resulting in extraluminal, persisting extravasation. The key efficacy measure was defined as successful CTO crossing using the Ocelot catheter as identified by guidewire placement in the distal true lumen and confirmed by angiography (allowing use of other crossing/assist/re-entry devices). Secondary efficacy measures included: use of re-entry/assist devices in lesions that were successfully crossed, procedure time (defined as initial stick to last wire out), CTO crossing time, fluoroscopy time, contrast dose and amount of saline flush (through Ocelot for visualisation only).
STATISTICAL ANALYSES
The resulting values are presented as mean ± standard deviation. Analyses comparing Ocelot MPT data to CONNECT study data were made using Fisher’s exact test for categorical data and Student’s t-test for independent groups for continuous data. Statistical analyses were performed using SAS for Windows software, version 9.1 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Thirty-three patients with confirmed CTO (99-100% stenosed by visual estimate) of SFA were treated in this study. Mean patient age was 70±9 years old and 62% of patients were male. Eighty-one percent of patients were prior smokers, 81% had a history of hypertension, and 70% had previous non-coronary interventions. Complete patient demographics and comorbidities are provided in Table 1.
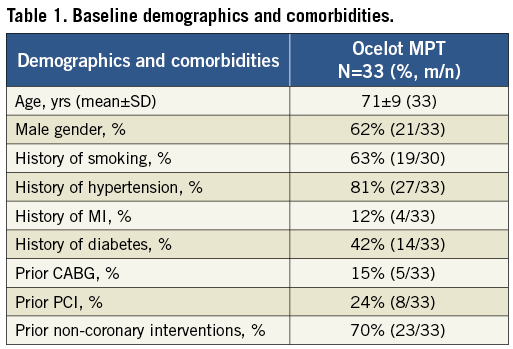
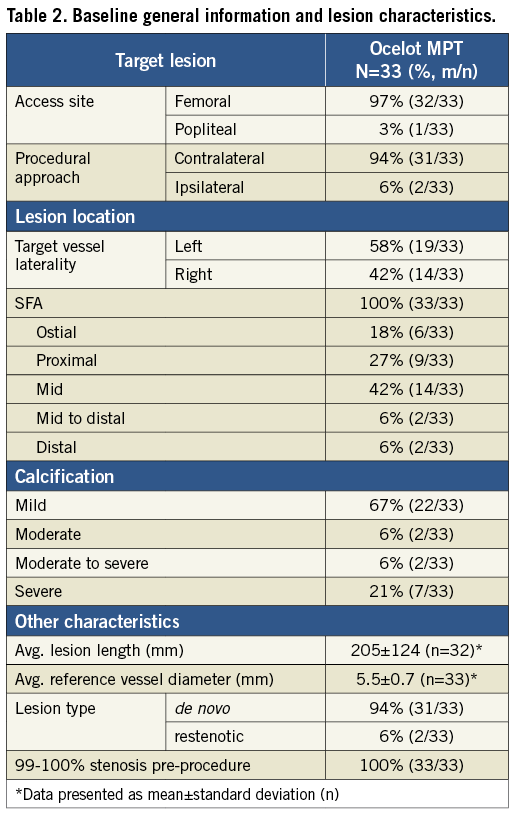
Baseline lesion characteristics are presented in Table 2. Of the 33 lesions, 18% were located at the ostium, 27% were proximal, 42% were mid, 6% were mid to distal and 6% were distal pertaining to the segment within the SFA. A mildly calcified lesion was identified in 67% of patients while 21% had severely calcified lesions. Mean lesion length and diameter by visual estimate were 205±124 mm and 5.5±0.7 mm, respectively. A de novo lesion was identified in 94% of patients.
PROCEDURAL RESULTS
All Ocelot MPT patients (100%) achieved the key safety measure, freedom from procedural MAE (clinically significant perforations, embolisations and grade C or greater dissections). Thirty-one out of 33 patients (94%) achieved the key efficacy measure, successful CTO crossing with the Ocelot system. In the two patients where the CTO was not successfully crossed, the lesions were identified as severely calcified along the entire SFA and subsequent attempts to cross the CTO with other crossing devices also failed (i.e., Wildcat; Avinger, Inc., Quick-Cross®; Spectranetics Corp., Colorado Springs, CO, USA, stiff guidewire, and Outback®; Cordis, Johnson & Johnson, Warren, NJ, USA). Both patients were subsequently referred for elective surgery. Key safety and efficacy results are provided in Table 3.
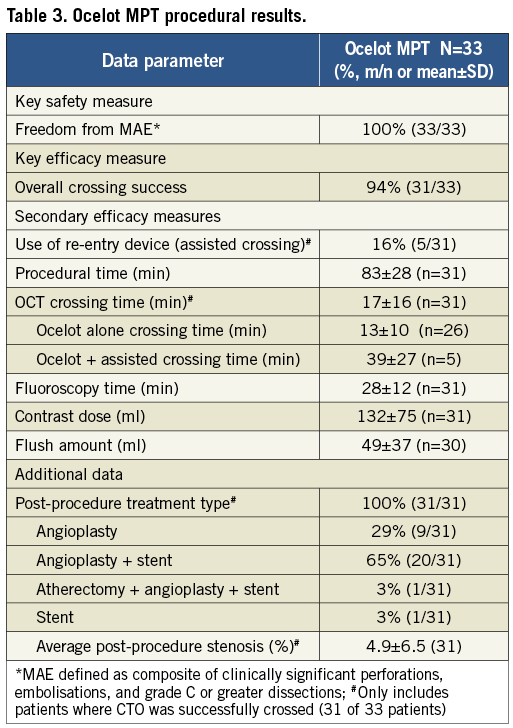
Ocelot MPT procedural results are provided in Table 4. Only five of 31 lesions successfully crossed (16.1%) required the use of a re-entry/assist device. The mean total procedure time was 83±28 minutes and the mean Ocelot catheter crossing time was 17±16 minutes for cases where the CTO was successfully crossed. While OCT visualisation was able to define the true lumen target site in these instances, the anatomic distortion of severely calcified distal caps proved prohibitive to catheter navigation, displacing the catheter into a subintimal plane requiring an assist device for re-entry. During two cases, the distal lesion cap was so severely calcified that true lumen re-entry was altogether unsuccessful. When no re-entry/assist devices were needed to cross the CTO (26/31), Ocelot crossing time decreased to 13±10 minutes. Mean contrast dose was 132±75 mL and mean flush amount was 49±37 mL. Subsequent therapies for those lesions crossed were consistent with standard EU practice and included: 29% angioplasty alone, 65% angioplasty and stent, 3% atherectomy, angioplasty and stent, and 3% treated with a stent alone. The average post-procedure stenosis was 4.9±6.5 percent, defined by angiographic imaging.
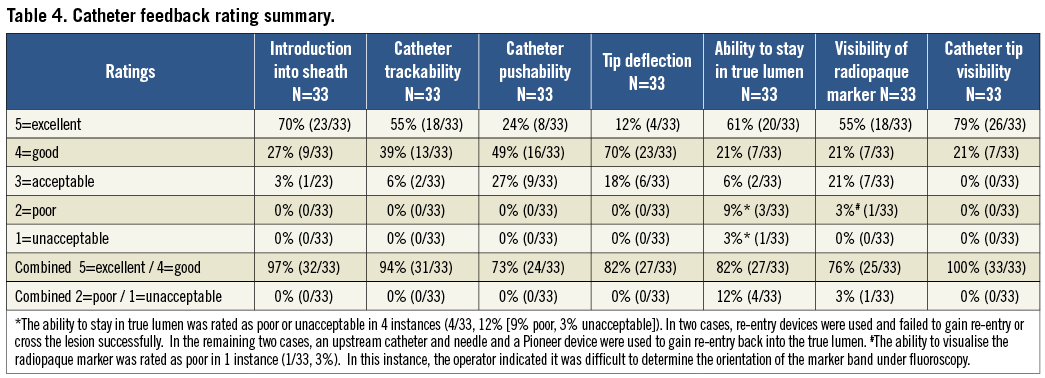
OCELOT CATHETER PERFORMANCE RATINGS
Overall physician feedback on the performance of the Ocelot catheter is presented in Table 5 and was positive, with an 87% average rating of excellent or good across the seven catheter categories. Catheter performance was physician-rated as excellent or good in the following percentages: 100% visibility of catheter tip under fluoroscopy, 97% catheter introduction into sheath, 94% catheter trackability, 82% ability to stay in true lumen, 82% tip deflection, 76% visibility of radiopaque markers, and 73% device pushability. The ability to stay in the true lumen was rated as poor or unacceptable in 4 instances (poor, n=3, and unacceptable, n=1). In two of the cases, re-entry devices were used and failed to gain re-entry or cross the lesion successfully and, in the remaining two cases, an upstream catheter and needle and a Pioneer® device (Medtronic CardioVascular, Santa Rosa, CA, USA) were used to gain re-entry back into the true lumen.
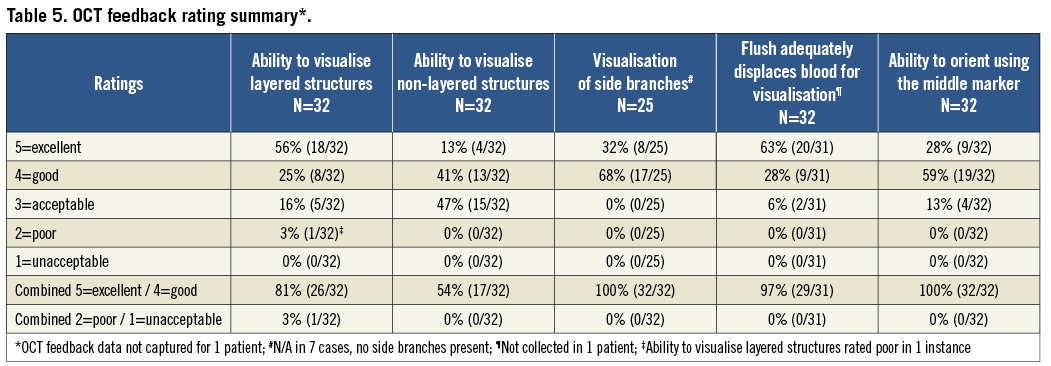
OCT PERFORMANCE RATINGS
Overall physician feedback on the performance of OCT is presented in Table 5 and was positive with an 86% average rating of excellent or good across the five OCT categories. OCT performance was physician rated as excellent or good in the following percentages: 100% visualisation of side branches, 100% ability to orient the device using the middle marker, 97% ability of saline flush to displace blood adequately for visualisation, 87% visualisation of vessel wall layered structures, and 54% visualisation of vessel wall non-layered structures. The only poor rating occurred in the category of ability to visualise the radiopaque marker. In this instance, the operator indicated that it was difficult to determine the orientation of the marker band under fluoroscopy.
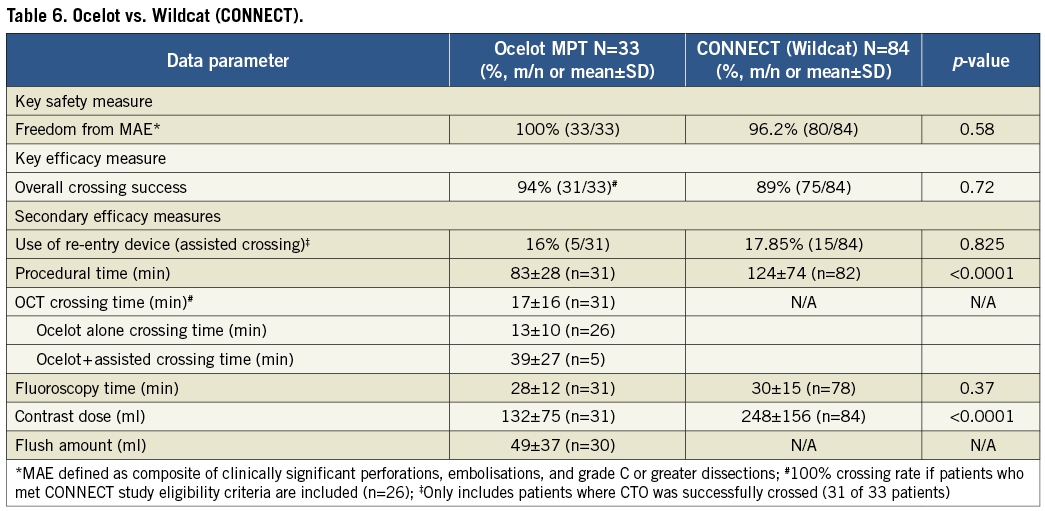
OCELOT COMPARED TO CONNECT (NON-OCT-GUIDED WILDCAT CATHETER)
Analyses comparing the OCT-guided Ocelot catheter to the non-OCT-guided Wildcat catheter (data collected in the CONNECT study16) are shown in Table 6. The key safety measure, freedom from clinically significant perforations, embolisations or grade C or greater dissections, was improved with the Ocelot, although not significantly (100% vs. 95.2%, p=0.58). The key efficacy measure, ability to cross the CTO, was also improved with the Ocelot as compared to the Wildcat, although again not significant (94% vs. 83%, p=0.72). When the ability to cross the CTO was analysed in only those Ocelot patients who met the inclusion and exclusion criteria of the CONNECT study (n=26), a 100% CTO crossing rate was observed (100% vs. 83%, p=0.11). Seven patients treated with the Ocelot would have been excluded from the CONNECT study due to a severely calcified target vessel.
Procedure time and contrast dose were significantly reduced with the use of the OCT-guided Ocelot catheter (Table 6). Procedure time decreased from 124±74 minutes with the Wildcat, to 83±28 minutes with the Ocelot, p<0.0001, while contrast dose decreased from 248±156 millilitres to 132±75 millilitres, respectively, p<0.001. There was not a statistically significant change in fluoroscopy time between the Ocelot (28±12 minutes) and Wildcat (30±15 minutes), p=0.37.
Discussion
CTOs represent the most technically challenging subset of PAD lesions. The current shift from primary surgical bypass to endovascular intervention is the result of improved technologies and post-crossing patency rates27,28. This may translate into shorter hospital stays, earlier patient recovery and ambulation, and reduced exposure to potential surgical and anaesthetic complications. Ultimately, the expanded availability of successful endovascular modalities for limb salvage and revascularisation has the potential to reduce associated healthcare costs, all the while improving patient satisfaction and outcomes30.
Currently available CTO crossing catheters utilise a variety of technologies. The CrosserTM CTO Recanalisation System (FlowCardia Inc.[Bard], Sunnyvale, CA, USA) transmits high frequency ultrasound mechanical vibrations to the distal tip with a reported recanalisation success rate of 75% (n=12) with no clinically significant perforations29. The rotational diamond-coated tip of the TruePathTM CTO device (Boston Scientific, Natick, MA, USA) demonstrated an 80% CTO crossing with 98.8% freedom from perforation (n=85)30. The Cordis Frontrunner XP (Cordis Corp., Miami, FL, USA) has a distal tip with hinged jaws, creating micro-dissections as it traverses CTOs. In one report of 26 SFA CTOs, 65% were successfully crossed32. Mossop et al reported a 91% success rate in 36 patients following failed guidewire crossing33. The CrossBossTM (BridgePoint, Plymouth, MN, USA), intended for coronary procedures, employs an atraumatic rounded tip with fast spin technique for crossing. The Wildcat (Avinger, Inc.) contains a distal element housing spiral wedges which, when engaged, actively corkscrew through CTOs with reported 89% efficacy (n=84)16.
IVUS and OCT are established diagnostic imaging modalities for both the peripheral and coronary vascular beds34. However, there are limited applications adapted for therapeutic intervention that would allow for comparison with the aforementioned non-imaging devices. Saket et al35 highlighted the CrossPoint TransAccess catheter (Medtronic Inc., Santa Rosa, CA, USA), a re-entry device with an intravascular ultrasound (IVUS) transducer for true lumen targeting. This technique was used successfully in seven patients following subintimal crossing and angioplasty.
When compared with IVUS, OCT’s infrared spectrum consistently demonstrates more reliable histopathological analyses of plaque morphology36. Coronary findings have been extrapolated to peripheral disease with OCT validations of histologic correlation via imagery. Therefore, OCT has been recognised as the technology that represents the most promising conduit for image-guided therapy with the advent of percutaneous therapies such as cutting balloon angioplasty, intravascular cryoablation, CTO crossing, and atherectomy37.
The Ocelot catheter, a new device aimed at crossing complex SFA CTOs, represents the first time that OCT has been incorporated into the distal tip of a peripheral CTO crossing catheter. The proprietary common path, OCT, enables the length variance and tip flexibility necessary for successful navigation of CTOs. Accordingly, the rotating optical fibre provides continuous A-scans, allowing for an extrapolated image that lends itself to real-time clinical decision making.
This paper reports on a multicentre European experience, where the Ocelot catheter was used to treat 33 patients with confirmed SFA CTOs with 100% freedom from major adverse events and 94% successful guidewire placement in the distal true lumen. Important secondary measures demonstrated statistically significant reductions in procedural time and contrast dose when compared to the Wildcat catheter, a non-OCT-guided CTO crossing catheter. While further study is needed to validate these findings, initial outcomes suggest an added benefit of healthcare cost savings related to shortened procedure time and improved patient safety with a reduction in delivered contrast volumes.
In accordance with previous investigational trials, difficulty with CTO crossing appeared to correlate with lesion-specific calcium burdens29. In the two patients where the CTO was not successfully crossed, the lesions were identified as severely calcified along the entire SFA and subsequent attempts to cross the CTO with other crossing devices also failed.
The Ocelot is a safe and effective tool moving forward in the treatment of complex femoropopliteal CTOs. The easy adoption and use of both the catheter and OCT technology will likely change the landscape of treating PAD. With an additional axis of imaging, Ocelot and OCT provide interventionalists with a safe, reliable, and reproducible tool for intraluminal CTO revascularisation and limb salvage.
Limitations
A few limitations of this study are: 1) small sample size; 2) non-randomised patient population; and 3) selection bias for patients with failed initial guidewire crossing of proximal cap, albeit unfavourable for the Ocelot cohort as this probably resulted in longer, more complex, and calcified lesions. Additional experience is needed to validate the use of this device in endovascular therapy and will be forthcoming via the CONNECT II study.
Conflict of interest statement
J.C. George, J. Pigott and G. Robertson are consultants for Avinger, Inc., and have investments in Avinger, Inc. M. Selmon and B. Reimers are consultants for Avinger, Inc. G. Shrikhander has received an educational grant from Avinger, Inc., and has investments in Avinger, Inc. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Animated picture of the Ocelot.

