Transcatheter aortic valve implantation (TAVI) has emerged as a major breakthrough in the field of interventional cardiology in the past few years and is now recognised and recommended as an alternative for selected patients with severe aortic stenosis (AS), particularly for those at high surgical risk1. The acknowledged benefits of TAVI include improved mortality rates, left ventricular (LV) haemodynamics and remodelling, NYHA functional class, and quality of life2. Aortic stenosis is characterised by progressive narrowing of the aortic valve which increases the pressure afterload on the left ventricle, inducing a hypertrophic response with increased wall thickness, enlarged cardiomyocytes and finally maladaptive transition from normal to impaired left ventricular function3. However, aortic valve stenosis might also have an impact on the peripheral vessels and is associated with the presence of a systemic endothelial dysfunction4. Previous works have reported evidence of abnormal endothelial function in patients with AS, as witnessed by the presence of blunted flow-mediated dilation (FMD) values or a higher concentration of circulating endothelial microparticles (two non-invasive indices of systemic endothelial function)4,5. Nevertheless, the exact nature of this relation is not clearly elucidated. On the one hand, this association might be a matter of hazard: endothelial dysfunction could be related to different factors such as older age, hypertension, presence of underlying coronary artery disease, peripheral artery disease, renal failure or congestive heart failure, which are frequently present in patients with severe AS6,7. On the other hand, AS could directly promote endothelial dysfunction: valve narrowing and reduced LV stroke volume lead to decreased systemic blood flow and cardiac output which, in turn, will directly affect endothelial shear stress, a crucial factor for endothelial cell survival and function (Figure 1). Hence, decreased shear stress has been shown to enhance endothelial cell death and vesiculation8 and to reduce systemic FMD9. The presence of an underlying endothelial dysfunction in AS patients is far from being trivial: endothelial function is widely acknowledged as a promoting factor for future adverse cardiovascular events, because of its impact on the regulation of coronary vasodilation, platelet function, systemic hypertension or vascular inflammation10. Thus, its presence might also account partially (at least as an additional risk factor) for the poor cardiovascular outcome of these patients with severe AS.
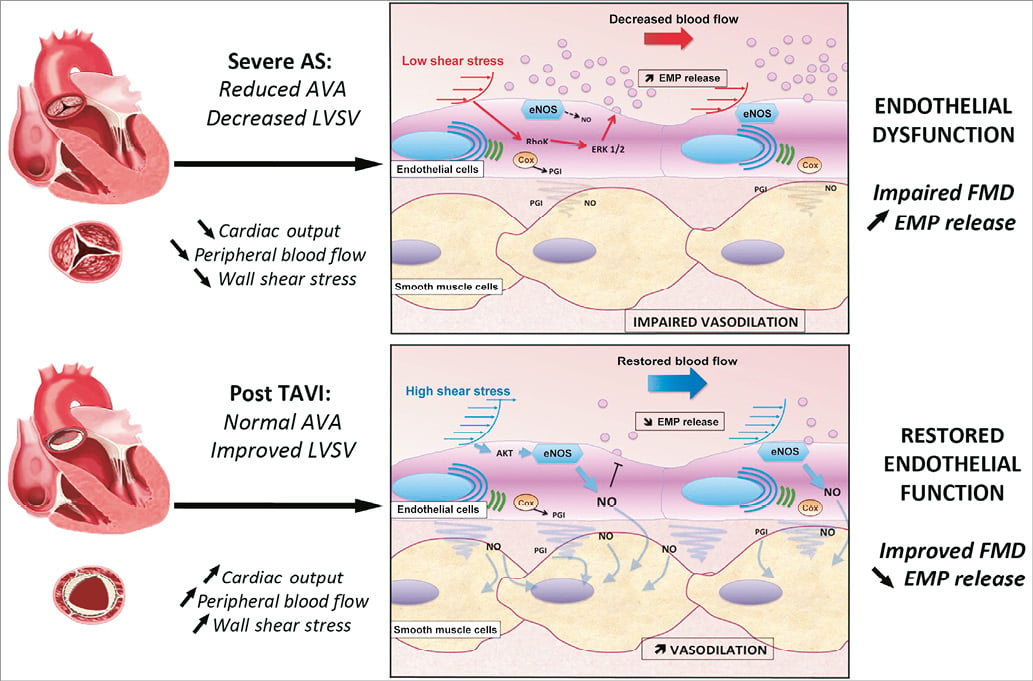
Figure 1. Potential mechanisms relating aortic stenosis and its correction to endothelial function. AS is characterised by reduced valve area and decreased LV stroke volume, which impairs cardiac output and peripheral blood flow. This condition is associated with lower values of wall shear stress, reduced production of NO by eNOS, as well as activation of the ERK1/2 and RhoK intracellular endothelial pathways leading to increased release of cellular microparticles. The result is a global endothelial dysfunction that is witnessed by blunted values of FMD. Correction of AS by TAVI procedure supports restoration of a normal peripheral blood flow and improved wall shear stress values. The higher local endothelial shear stress promotes activation of eNOS through the Akt pathway, leading to increased NO production and decreased endothelial MP release. Enhanced NO release will improve smooth muscle cell vasodilator competences, leading to improved FMD values. AS: aortic stenosis; AVA: aortic valve area; Cox: cyclo-oxygenase; EMPs: endothelial microparticles; eNOS: endothelial nitric oxide synthase; FMD: flow-mediated dilation; LV: left ventricle; LVSV: left ventricular stroke volume; NO: nitric oxide; PGI: prostaglandins
In this context, the present study by Horn and colleagues gives us a new perspective on the relationship between aortic stenosis and endothelial dysfunction11. In this clinical cohort, the authors observed that TAVI procedures led to an improvement of wall shear stress and brachial artery flow velocity, confirming the favourable impact of the procedure on the systemic blood flow. The endothelial function was significantly improved, as witnessed by higher FMD values and lower levels of circulating endothelial microparticles compared to baseline, although the pre-existing cardiovascular risk factors and comorbidities that could have impaired endothelial function were still present in the patients. In addition, the authors reported that the main predictor of FMD values in patients before TAVI was the baseline wall shear stress values, independently of other traditional risk factors. Altogether, these data suggested that AS per se was the main cause of the endothelial dysfunction in these patients, probably through the impact of decreased cardiac output and reduced shear stress mechanism on endothelial cells (Figure 1).
However, this study also raises some new issues: we do not know if the observed results were consistent in all patients or if they depended on the baseline left ventricle function characteristics. Was the increase in cardiac output and shear stress related solely to the enlargement of the effective aortic valve area or was there an influence of improved LV function? Do the patients with poor LVEF and/or low flow have comparable evolution to the others, since the myocardial recovery process appears to be different among these groups12? Finally, how can we explain the discordance between the conclusions of this study and a previously published smaller series in which the authors did not observe any improvement of endothelial function following surgical aortic valve replacement13? Can the different baseline characteristics of the patients (especially their arterial mechanical characteristics) account for these discrepancies as suggested by Horn and colleagues11? Or, is there a deleterious role of heart surgery and cardiopulmonary bypass on endothelium which thwarts the benefit of aortic valve replacement, as suggested by previous studies14?
Many of the aforementioned points should be addressed in future studies, since we should now consider AS not only as a “disease of the valve and the myocardium”3, but also involving peripheral arteries. Hence, TAVI could be envisaged as a therapy for both heart and the vessels, and its benefits for cardiovascular outcome could eventually be at least partially mediated by improvement of the systemic endothelial function.
Conflict of interest statement
C. Caussin is a proctor for Edwards Lifesciences and Medtronic. C.M. Boulanger and N. Amabile have no conflicts of interest to declare.

