Abstract
Aims: Degenerative aortic valve stenosis (AVS) is independently associated with endothelial dysfunction and increased levels of circulating endothelium-derived microparticles (EMPs) as a marker of compromised endothelial integrity. The aim of this study was to investigate whether therapy for severe AVS by transcatheter aortic valve implantation (TAVI) improves endothelial function and decreases EMPs.
Methods and results: Fifty-six patients with indication for TAVI due to symptomatic severe AVS were prospectively enrolled. Brachial wall shear stress (WSS), endothelial function and circulating microparticles (MPs) were measured before and three months following TAVI. Endothelial function was assessed as flow-mediated dilation (FMD) using ultrasound. MP subpopulations were discriminated by flow cytometry according to the expression of established surface antigens: CD31+/CD41–, CD144+ and CD62E+ as EMPs and CD41+ as platelet-derived MPs (PMPs). In patients with severe AVS, decreased brachial WSS was an independent predictor of low FMD. At three-month follow-up after TAVI, WSS and FMD increased along with decreased levels of EMPs as compared to pre TAVI. Decrease of CD31+/CD41–, CD144+ and CD62E+ EMP levels correlated with the increase of FMD.
Conclusions: Therapy for AVS by TAVI was associated with improved endothelial function and integrity indicating beneficial effects of TAVI on systemic arterial function.
Introduction
Degenerative aortic valve stenosis (AVS) is the leading cause of aortic valve morbidity with a prevalence of 2-7% at ages above 65 years and an even further increasing incidence in the eighth decade1. AVS is independently associated with traditional cardiovascular risk factors and clinically apparent CV disease2, indicating that the degeneration of the aortic valve may represent an atherosclerosis-like process involving both the aortic valve as well as the vascular system3. In addition, AVS alters systemic haemodynamics through a reduced stroke volume and an impaired pulsatile flow, which might affect WSS along the vascular wall of conduit and resistive arteries. The vascular endothelium is exposed to various mechanical forces such as blood pressure and WSS which are able to influence vascular tone4. Maintenance of a flow-mediated physiological laminar WSS is crucial for normal vascular structure and function through the release of several vasodilators, in particular nitric oxide (NO)5 and prostaglandins (PGI2)6 mediating flow-mediated vasodilation (FMD). In patients with AVS, downstream in peripheral arteries WSS is decreased7, FMD is impaired indicating endothelial dysfunction8,9, and levels of circulating endothelial microparticles (EMPs) are increased10. EMPs are membrane vesicles of less than one micrometre in diameter, which are shed from activated and apoptotic endothelial cells into the circulation. EMPs can be considered as circulating markers of a compromised endothelial integrity11,12. Circulating levels of EMPs increase early during atherosclerotic processes, correlate with the degree of endothelial dysfunction13, and have been established as prognostic biomarkers predicting adverse cardiovascular outcome14,15.
It is still unknown whether in AVS the endothelial function is impaired as a consequence of the atherosclerotic process itself, due to changes of driving mechanical forces such as WSS downstream of the valve, or secondary to other factors beyond physical pressure effects. We hypothesised that altered haemodynamics may at least partly be responsible for the endothelial dysfunction observed in patients with AVS. Therefore, the aim of this study was to investigate whether treatment of severe AVS by transcatheter valve implantation (TAVI) may increase peripheral arterial WSS, improve endothelial function and decrease levels of circulating EMPs as markers of compromised endothelial integrity.
Methods
STUDY DESIGN
In this prospective observational study a total of 61 patients with symptomatic severe AVS scheduled for TAVI were included. Severe AVS was defined according to the guidelines of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery16. Transvalvular pressure gradients, peak velocity and valve area were assessed by transthoracic echocardiography as well as cardiac catheterisation. Patients were considered unsuitable for conventional aortic valve replacement according to the University of Duesseldorf Heart Team due to severe comorbidities and frailty (Table 1).
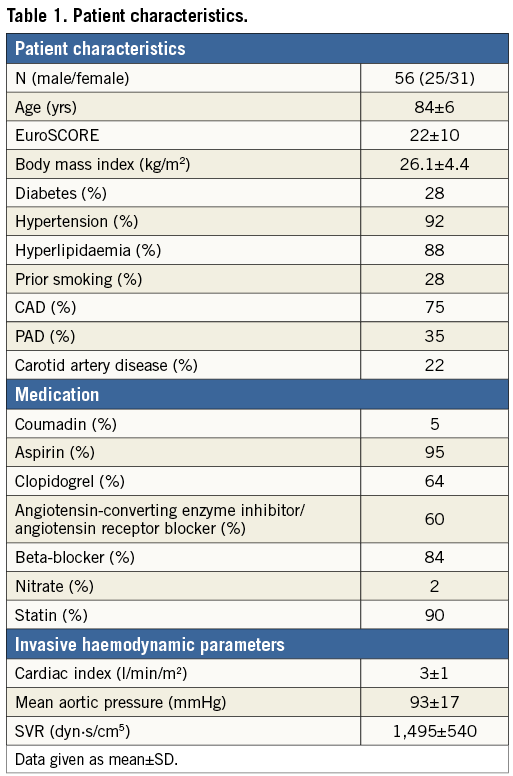
Of 61 patients included in the study, four died and one discontinued the follow-up due to hospitalisation (Figure 1A). Physicomechanical properties affecting endothelial cells such as WSS, pulse pressure, arterial stiffness, and indices of endothelial function and integrity such as FMD and plasma levels of circulating microparticles (MPs) were measured at the time of diagnosis of the severe AVS pre TAVI and at a three-month study point post TAVI (Figure 1B).
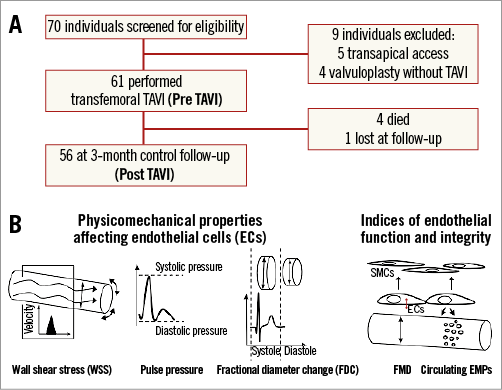
Figure 1. Study protocol. A. Flow of participants through the phases of the study. B. Pre- and post-TAVI physicomechanical properties affecting endothelial cells (ECs) were assessed as wall shear stress (WSS), pulse pressure and arterial stiffness by measuring the fractional diameter change (FDC). Indices of endothelial function and integrity were assessed as flow-mediated dilation (FMD) and plasma levels of endothelial microparticles (EMPs). SMC: smooth muscle cell
General exclusion criteria were the presence of heart rhythms other than sinus rhythm, recent or current inflammatory condition, stage 5 chronic kidney disease, and active malignancies within the last year. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects and patients were approved by the University of Duesseldorf Committee on Human Research (ClinicalTrials.gov: NCT01993485). Written informed consent was witnessed and formally recorded.
TAVI PROCEDURE
All TAVI procedures were performed by femoral artery access. During the catheterisation ascending aortography and bilateral iliofemoral arteriography were performed. During the procedure, an 18 Fr delivery sheath (Medtronic, Minneapolis, MN, USA) was placed into the femoral artery. A temporary pacemaker was placed in the right ventricular apex, and a balloon valvuloplasty was performed under rapid ventricular pacing followed by implantation of the valve (CoreValve; Medtronic). The femoral artery access was then closed by a closure device (Prostar XL; Abbott Vascular, Santa Clara, CA, USA). All patients received local anaesthesia and deep sedation only. Eleven patients (21%) received blood transfusions post procedure.
PHARMACOLOGICAL REGIMEN POST TAVI
All individual medications did not change throughout the study period except for clopidogrel which was added during the TAVI procedure in all patients who were not already receiving clopidogrel. The standard regimen after TAVI in our centre consists of aspirin plus clopidogrel for three months, followed by monotherapy with aspirin. Sixty-four percent of our patients were already on aspirin plus clopidogrel before the TAVI procedure due to recent coronary intervention. Therefore, the antiplatelet regimen changed in 36% of our study patients.
ASSESSMENT OF ENDOTHELIAL FUNCTION
Endothelial function was assessed by measuring FMD of the brachial artery by ultrasound (Vivid i; GE Healthcare, Munich, Germany). Baseline data for diameter and mean blood flow velocity of the brachial artery were quantified after 20 min of supine rest in an air-conditioned room (21°C) at 1 to 2 cm above the elbow following an identical protocol. The image and flow analyses were performed offline from recorded loops with an automated system (Brachial Analyzer 5; Medical Imaging Applications, Coralville, Iowa City, IA, USA). All diameter readings were taken at diastole. Flow velocity represents the mean angle-corrected Doppler flow velocity. Vasodilation results are presented as percent change: (Diameterpostischaemia - Diameterbaseline/Diameterbaseline)×100. Assessment of endothelium-independent vasodilatation with nitroglycerine was not performed due to safety concerns regarding the risk of severe hypotension in patients with critical AVS.
CALCULATION OF WALL SHEAR STRESS (WSS)
The image and flow analyses were performed offline from recorded loops with an automated system (Brachial Analyzer 5; Medical Imaging Applications). All diameter readings were taken at diastole. Flow velocity represents the mean angle-corrected Doppler flow velocity. Flow was calculated as π×(Diameter/2)×2×flow velocity (V). Hyperaemic blood flow after occlusion of the forearm increases WSS in the brachial artery and the increase in WSS represents the stimulus for FMD. The WSS was calculated as: 8×µ×V/Diameter, where blood viscosity (µ) was assumed to be constant at 0.035 dyne×s/cm217.
ASSESSMENT OF LOCAL VASCULAR STIFFNESS
Images for the measurement of fractional diameter change (FDC) of the brachial artery were assessed during the same FMD session as previously described18. In order to evaluate FDC, a scan was made in a longitudinal section with attention to a clear differentiation of the intima-media complex of the anterior and posterior wall. FDC was determined during one heart cycle and calculated as the difference between maximum systolic diameter and the minimum diastolic diameter in relation to the minimum diastolic diameter using an automated analysis system (Brachial Analyzer; Medical Imaging Applications).
ASSESSMENT OF CIRCULATING MPS AS A MARKER FOR ENDOTHELIAL INTEGRITY
MP subpopulations were discriminated by flow cytometry according to the expression of established surface antigens as described previously19. Briefly, citrated blood (6 ml) was drawn from the cubital vein. Platelet-free plasma was obtained by successive centrifugations of the supernatants at 300 g and 10,000 g for five minutes at room temperature. Platelet-free plasma (PFP) obtained by this sequential centrifugation contains MPs but not platelets, as shown by flow cytometry and fluorescence-based laser-scanning microscopy19. PFP were stored at –80°C. Samples of all patients were handled the same way regarding sample preparation, storage, and performance of flow cytometry. PFP were incubated for 30 minutes with fluorochrome-labelled antibodies or matching isotype controls and analysed in a Canto II flow cytometer (Becton Dickinson, Heidelberg, Germany). The events were discriminated by 1.0 µm microbead standards (Polysciences Inc., Eppelheim, Germany). EMP subpopulations were defined as CD144+, CD62E+ or CD31+/CD41–. Platelet-derived MPs (PMPs) were defined as CD41+ MPs. The total number of MPs was quantified with flow count calibrator beads (20 µl; Beckman Coulter, Brea, CA, USA). Unless specified otherwise, chemicals were purchased from Sigma-Aldrich (Deisenhofen, Germany).
Statistics
Results are expressed as means±standard deviation (SD) of the mean. Patients’ characteristics were analysed using paired t-tests. The D’Agostino-Pearson omnibus normality test was used to confirm normal distribution of haemodynamic parameters and FMD values and non-normal distribution of EMP levels. The Student’s t-test was used to analyse the effect of intervention on haemodynamic parameters. The Wilcoxon matched pairs test was used to analyse the effect of the intervention on EMP levels. Correlation of haemodynamic parameters was assessed by Pearson coefficient, and correlation of EMPs was assessed by Spearman coefficient. P-values <0.05 were considered as statistically significant. A multivariate regression model was used to determine the influence of multiple parameters on FMD. Variables for the linear regression model were chosen based on simple correlation analysis and those variables known or thought to be associated with endothelial function. Only variables with p<0.1 in univariate analysis were included in multivariate analysis. All statistical tests were conducted using SPSS 21.0 (IBM, Armonk, NY, USA) and Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Results
CHARACTERISTICS OF STUDY GROUP
Four patients died during the first 24 hrs post intervention due to vascular complications (n=3) or a rupture of the aortic annulus (n=1). The pre-TAVI FMD of these patients was rated in the median range of the total cohort and we did not see a relationship between pre-TAVI FMD and complications/prognosis in this study. The followed-up study group consisted of 56 elderly patients with a mean EuroSCORE of 22 indicative of the severe comorbidities (Table 1). Pre-TAVI FMD (3.2±0.9%) was lower compared to age-matched patients (n=20) without aortic valve stenosis (4.0±1.0%, p=0.002) (data not shown). The TAVI procedure was successfully performed in all patients as mean transvalvular pressure gradients decreased from 42±18 mmHg pre TAVI to 7±4 mmHg post TAVI (p<0.0001), and mean transvalvular peak velocity decreased from 4.1±0.8 m/sec to 1.7±0.4 m/sec (p<0.0001) (Table 2).
WALL SHEAR STRESS PREDICTS FMD IN PATIENTS WITH AVS PRE TAVI
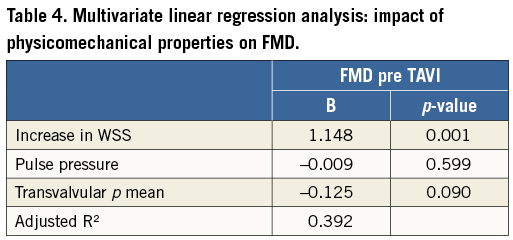
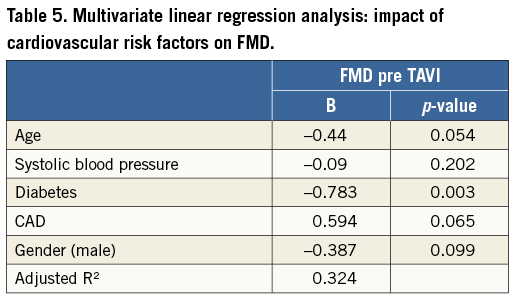
Pre TAVI, pulse pressure as well as the increase in WSS from baseline after induction of hyperaemia correlated with FMD (Table 3). In multivariate regression analysis the increase in WSS was an independent predictor of FMD in patients with AVS in a model including the physicomechanical properties pulse pressure and transvalvular pressure gradient (95% CI: 0.623 to 1.616, p=0.001) (Table 4). In this elderly population, diabetes was the only cardiovascular risk factor that affected FMD in a multivariate regression model (Table 5). Detailed non-standardised coefficients and significance levels are given in Table 4 and Table 5.
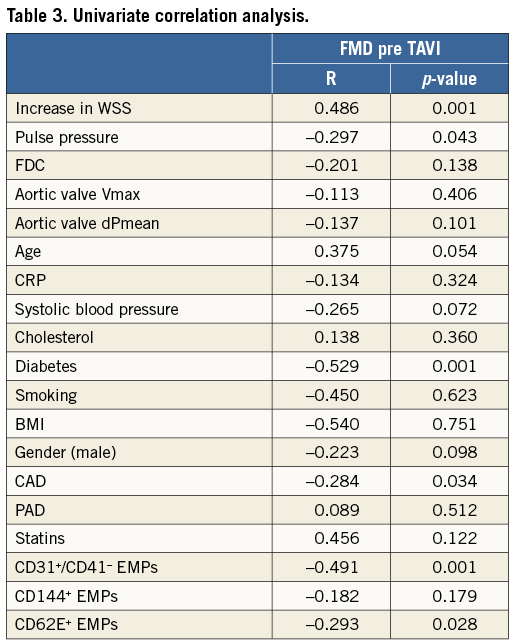
In patients with AVS, pre-TAVI FMD values correlated with levels of CD31+/CD41- EMPs (r=0.491, p=0.001) and CD62E+ EMPs (r=-0.293, p=0.028) (Table 3).
INCREASED WALL SHEAR STRESS AND FMD POST TAVI
In the brachial artery, the increase in WSS from baseline rose from 0.5±0.5 s–1 pre TAVI to 0.7±0.4 s–1 post TAVI (Figure 2A). FMD measured in the brachial artery increased from 3.2±0.9% pre TAVI to 3.6±0.8% post TAVI (p=0.01) (Figure 2B). The increase in FMD pre TAVI to post TAVI correlated with the increase in delta WSS from baseline (r=0.36, p=0.0058) (Figure 2C). The increase in FMD pre TAVI to post TAVI correlated (not significantly) with the decrease in mean aortic valve pressure gradients (r=-0.266, p=0.051).
Pulse pressure did not change after TAVI (pre TAVI 66±9 mmHg, post TAVI 69±10 mmHg, p=0.1512) (Figure 2D). Within three months TAVI did not affect indexes of arterial stiffness and compliance (FDC) in conduit arteries (0.023±0.015 pre TAVI compared to 0.022±0.015 post TAVI; p=0.5784) (Figure 2E).
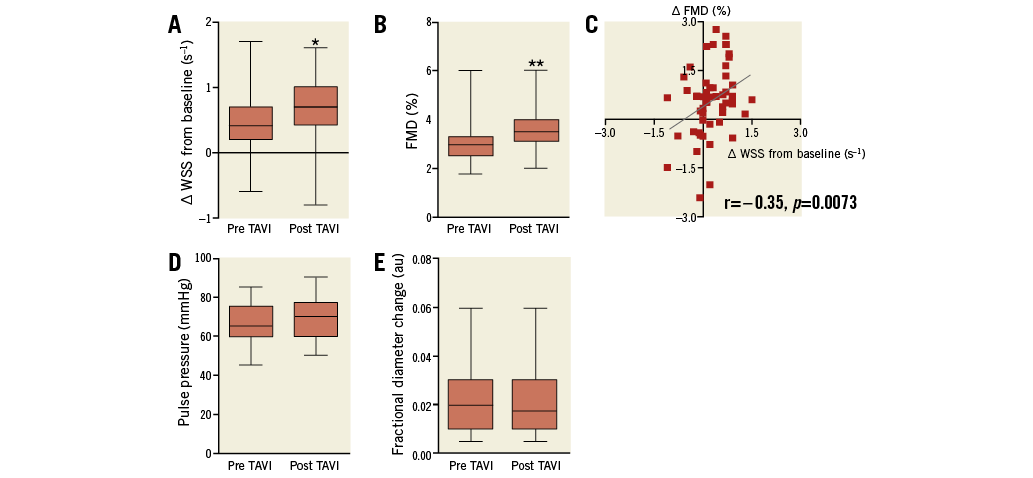
Figure 2. Wall shear stress (WSS) and flow-mediated dilation (FMD) increased post TAVI. A. The increase in WSS from baseline after induction of hyperaemia increased at three-month follow-up post TAVI compared to pre TAVI. B. FMD increased post TAVI compared to pre TAVI. C. The increase in FMD correlates with the increase in WSS from baseline. D. Pulse pressure and (E) fractional diameter change (FDC) did not change. *indicates p<0.05, **p<0.01 compared to pre TAVI
LEVELS OF EMPS DECREASED POST TAVI AND CORRELATED INVERSELY WITH FMD
Levels of CD31+/41– EMPs decreased from 258±143/µl pre TAVI to 216±130/µl post TAVI (p=0.0357) (Figure 3A). Levels of CD144+ EMPs decreased from 349±281/µl pre TAVI to 275±295/µl post TAVI (p=0.0008) (Figure 3C), and levels of CD62E+ EMPs also decreased from 1,218±786/µl pre TAVI to 960±660/µl post TAVI (p=0.026) (Figure 3E). Our data show a correlation between the increase of FMD and the decrease of EMP levels (post-TAVI – pre-TAVI): delta FMD with delta CD31+/41– EMPs (r=–0.31, p=0.0187) (Figure 3B), with delta CD144+ EMPs (r=–0.44, p=0.0007) (Figure 3D) and with delta CD62E+ EMPs (r=–0.36, p=0.0058) (Figure 3F).
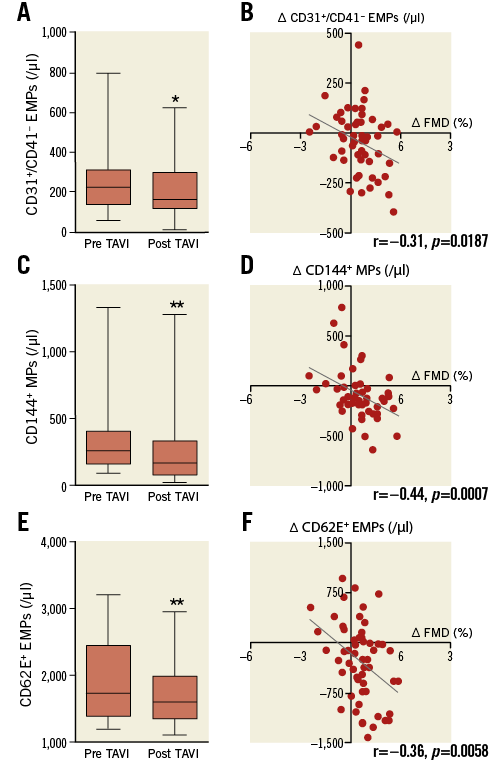
Figure 3. Levels of EMPs decreased after TAVI. Levels of EMPs as a marker of compromised endothelial integrity decreased at three-month follow-up post TAVI compared to pre TAVI. Levels of EMPs were measured as (A) CD31+/41+, (C) CD144+ and (E) CD62E+ MPs. * indicates p<0.05, ** p<0.01 compared to pre TAVI. Increase of FMD post TAVI compared to pre TAVI correlates with the decrease of (B) CD31+/41+, (D) CD144+ and (F) CD62E+ EMP levels.
To test whether the effects observed were specifically related to endothelial cells or extended to other non-endothelial MP populations, we measured the levels of PMPs. The levels of PMPs were not affected by the TAVI procedure: 2,807±3,465/µl pre TAVI compared to 2,887±3,340/µl post TAVI (p=0.8831) (data not shown).
Discussion
Here we show in elderly patients with severe AVS that brachial WSS is an independent predictor of FMD. Following TAVI, brachial WSS increased, FMD improved and levels of EMPs decreased, indicating that TAVI restored endothelial integrity. Improved stroke volume and pulsatile flow pattern due to replacement of obstructed aortic valves may add to the beneficial effects of TAVI on systemic arterial function.
AORTIC VALVE STENOSIS AND ENDOTHELIAL FUNCTION: IMPACT OF TAVI
Irace et al demonstrated that patients with AVS have lower levels of WSS (in the common carotid artery) compared to patients without AVS, and that conventional aortic valve replacement (AVR) increased blood velocity leading to increased baseline WSS7. These results were in line with the increased WSS after TAVI in our study which might contribute to the improved endothelial function after TAVI. In contrast to our results, in a small study of 15 patients with AVS undergoing conventional AVR, Chenevard et al demonstrated that endothelial function (FMD) did not improve20. The patients included in our study differed from that study as they were much older, had more cardiovascular risk factors and severe comorbidities. Presumably they had stiffer and more calcified arteries and the diameter did not change after resolving the AVS in contrast to the decrease in arterial diameter post AVR measured in the study of Chenevard et al. As the brachial diameter in our study did not change, the increase in peak flow velocity yielded higher WSS as the stimulus for FMD. The patients included in our study were also more limited in daily exercise due to the AVS pre TAVI compared to the younger patients undergoing AVR, so that the effect of the recovery of exercise capacity on endothelial function after resolution of AVS might be more pronounced in our patients.
In our study, baseline brachial diameter, pulse pressure, as well as FDC indicating arterial stiffness of the muscular peripheral arteries, remained unchanged by TAVI. Previously it was shown that aortic valve replacement did not change pulse pressure21, but was associated with an improvement in aortic stiffness at long-term follow-up (12 months), although not in the short term (one to six months)21. Therefore, our results could be explained by a relatively short observation period of three months which might be too short to show an effect on arterial stiffness.
Taken together, we here show that in elderly patients with AVS WSS is an independent predictor of pre-TAVI FMD, and that WSS and FMD increased following TAVI.
AORTIC VALVE STENOSIS AND ENDOTHELIAL INTEGRITY: IMPACT OF TAVI
We demonstrated that levels of EMPs decreased along with the improvement in endothelial function after TAVI. The precise mechanisms leading to MP generation by endothelial cells have not yet been validated in vivo. The current knowledge on MP formation derives mainly from experiments on isolated or cultured cells, showing that both cell activation and cell apoptosis can induce MP release by increasing intracellular calcium, loss in membrane lipid asymmetry, and cytoskeleton protein reorganisation12. WSS is the major determinant of endothelial apoptosis, and physiological laminar fluid WSS promotes endothelial cell survival and senescence22. In vivo, WSS correlated inversely with levels of EMPs23,24. In vitro, sustained atheroprone low WSS stimulated EMP release through activation of Rho-associated protein kinase and mitogen-activated protein kinase pathways, whereas atheroprotective high WSS limits EMP release24. These findings identified WSS as a physiological regulator of EMP formation and release24. WSS is reduced in patients with AVS7, and Diehl et al previously showed increased levels of circulating EMPs in patients with severe AVS10. We demonstrated that following TAVI endothelial function increased while levels of circulating EMPs decreased, along with increased brachial WSS. This indicates that recovered WSS after TAVI might prevent the release of MPs by protecting endothelial cells.
Study limitations
The study is limited due to the lack of an untreated control cohort not undergoing the TAVI procedure. Unfortunately, such a control cohort is unavailable, given the fact that patients with symptomatic severe AVS exhibit a short life expectancy prognosis and that the TAVI procedure was found to be superior to optimal medical therapy in reducing morbidity and mortality in this cohort25. Therefore, virtually all patients in our clinic with symptomatic severe AVS and severe comorbidities are referred for TAVI according to a Heart Team decision. Likewise, patients rejected for TAVI do not match our study cohort due to more severe comorbidities and/or the presence of malignancy.
An impact of the changes performed in antiplatelet regimen (in 36% of our study patients) on FMD or levels of EMPs could not be excluded in this study. It has previously been shown that clopidogrel reduced formation of EMPs in vitro26, improved FMD27 and reduced levels of circulating EMPs28 in vivo as an acute effect after a single dose, but clopidogrel did not affect FMD or EMP levels in the long term (30 days and three weeks, respectively) in vivo29,30. Therefore, we suppose that the change in antiplatelet regimen did not account for the alterations detected here.
Conclusion
Taken together, our data show that therapy of AVS was associated with improved endothelial function and integrity, indicating beneficial effects of TAVI on systemic arterial function in patients with severe AVS.
| Impact on daily practice This study indicates that TAVI in patients with severe aortic valve stenosis may exert beneficial effects beyond the heart, on the vascular system as indicated by ameliorated FMD and reduced numbers of endothelial microparticles following TAVI. Future studies with larger patient cohorts are required to decipher the potential prognostic value of the pre-existing endothelial dysfunction and its potential to recover after TAVI. In daily TAVI practice the assessment of endothelial function pre and post TAVI might be implicated in risk stratification. |
Acknowledgements
The study was supported with a restricted grant from the federal state government of North Rhine-Westphalia and the European Union (EFRE-Program “Med in NRW”, support code 005-GW01-235A). Flow cytometry facility was provided by the Susanne Bunnenberg Foundation at the Duesseldorf Heart Center.
Conflict of interest statement
The authors have no conflicts of interest to declare.

