
Abstract
Aims: The aim of this study was to examine the mechanisms of cognitive impairment and reversibility in elderly patients with severe aortic stenosis (AS) after transcatheter aortic valve implantation (TAVI) with special reference to cerebral blood flow (CBF).
Methods and results: We examined 15 elderly patients with severe AS (mean age 83.2±4.5 years, 12 female) who underwent TAVI. Before and three months after TAVI, we evaluated cognitive function with the Logical Memory II test (LM II), cardiac output (CO) with echocardiography, and CBF with 99mTc single-photon emission computed tomography (SPECT). LM II score and CO were significantly increased after TAVI compared with baseline (p<0.01 for LM II, p<0.005 for CO). Notably, CBF in the local regions, including that in the right hippocampus, was significantly increased after TAVI (p<0.005 at each voxel). The patients with increased CO after TAVI also showed significantly increased CBF in the right hippocampus compared with those without it (p<0.01). Importantly, CBF in the right hippocampus was positively correlated with LM II scores (p<0.05).
Conclusions: These results provide the first evidence that TAVI may improve cognitive functions associated with increased cerebral perfusion especially in the hippocampus in elderly patients with severe AS.
Introduction
Severe aortic valve stenosis (AS) is the most common valvular heart disease in the elderly in Western countries and Asia that gradually leads to progression of valve calcification and eventually causes heart failure1,2. The interaction between the heart and the brain is important in the elderly with multiple comorbidities3, because the two important organ systems share many pathophysiological mechanisms3. Indeed, cognitive impairment is frequently noted in patients with AS4,5,6. Although severe AS is conventionally treated with surgical aortic valve replacement, the less invasive transcatheter aortic valve implantation (TAVI) has been developed for such elderly frail patients at high surgical risk7.
Previous studies have examined cognitive functions and diffusion-weighted magnetic resonance imaging (DW-MRI) in patients with severe AS who underwent TAVI4,8. Notably, recent studies have demonstrated that some patients with severe AS showed improved cognitive functions after TAVI5,9. However, detailed mechanisms for the improvement after TAVI remain to be examined. Notably, cerebral perfusion has been regarded as an important pathophysiological factor of the heart and brain interactions3. We and others have previously demonstrated that brain perfusion single-photon emission computed tomography (SPECT) is a useful imaging technique to evaluate regional cerebral perfusion and its relevance to cognitive impairment or stress cardiomyopathy10,11.
In the present study, we tested our hypothesis that TAVI increases CBF associated with increased cardiac output (CO) with a resultant improvement of cognitive functions in elderly patients with severe AS, using brain perfusion SPECT imaging before and three months after TAVI.
Methods
The present study protocol was approved by the ethics committee of the Tohoku University Graduate School of Medicine (No. 2018-1-329) and was performed in compliance with the Declaration of Helsinki (UMIN000034203).
STUDY PATIENTS
From January 2017 to September 2018, we examined 57 consecutive patients with severe AS at the Tohoku University Hospital as candidates for TAVI. Inclusion criteria were 1) heart failure with New York Heart Association (NYHA) functional Class II to III symptoms, and 2) patient consent to undergo cognitive function tests for at least one hour. Exclusion criteria were 1) acute decompensated heart failure and heart failure with NYHA Class IV symptoms, 2) refusal of cognitive tests, and 3) insufficient quality of 99mTc SPECT. Based on these criteria, we excluded 35 patients in advance, including acute decompensated heart failure and heart failure with NYHA Class IV symptoms in 15, and refusal to undergo cognitive function tests for at least one hour in 20. Thus, we initially included 22 patients, seven of whom were then excluded because of dropout owing to refusal to undergo follow-up cognitive tests (n=2), and insufficient quality of 99mTc SPECT (n=5). Finally, we enrolled 15 patients in the present study with special reference to the association of cerebral blood flow (CBF) with cognitive functions (Supplementary Figure 1). Before and three months after TAVI, we measured cognitive functions with the Logical Memory II test (LM II)12, Mini-Mental State Examination (MMSE)13, and the Geriatric Depression Scale (GDS)14, CO with echocardiography, and CBF with 99mTc SPECT.
The baseline, TAVI procedure, and follow-up data were all collected in a dedicated database. Details of the TAVI procedure are shown in Supplementary Appendix 1.
ECHOCARDIOGRAPHY
The details of echocardiography are shown in Supplementary Appendix 2.
CBF IMAGE ACQUIREMENT AND PRE-PROCESSING
CBF can be measured not only by SPECT but also by MRI15,16. Since we and others have previously demonstrated that SPECT is a useful imaging technique to evaluate regional cerebral perfusion and its relevance to cognitive impairment or stress cardiomyopathy10,11, we selected SPECT for measuring CBF. H. Suzuki, who was blinded to the results of the imaging studies before and three months after TAVI, analysed and reported the SPECT scans. 99mTc-SPECT CBF images were acquired with a dual-head gamma camera (Symbia E; Siemens Healthineers, Erlangen, Germany). The following CBF image pre-processing and analyses were performed using SPM 12 software17. First, before CBF image analysis, we co-registered CBF images at three months to their corresponding baseline images. Second, the baseline and co-registered CBF images were normalised to the standard Montreal Neurological Institute space, using the SPECT template available in SPM 12. Finally, the normalised images were smoothed with an isotropic Gaussian kernel by convolving a 12 mm full width at half maximum to produce CBF maps. These pre-processing steps were described in detail in our previous reports16,18.
ASSESSMENT OF COGNITIVE FUNCTIONS
A standardised cognitive assessment with the LM II, MMSE, and GDS was performed by a single experienced staff member blinded to the results of the imaging studies before and three months after TAVI. The LM subtest of the Wechsler Memory Scale-Revised is internationally used as an operational definition to identify individuals with mild cognitive impairment (MCI). In particular, the LM II test (a 30-minute delayed test of prose recall) is an indicator to discriminate between healthy older adults and individuals with very mild cognitive impairment12. MMSE is a widely used screening tool for cognitive impairment13. GDS is a screening instrument for late-life depression that demonstrates good accuracy14. In addition, GDS is based mainly on behavioural and cognitive aspects of depression and is not heavily weighted towards somatic complaints14. Thus, GDS is supposed to differentiate depressed from non-depressed elderly adults suffering from physical illness reliably.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD). Normality was assessed using the Shapiro-Wilk test. Continuous variables were compared by the Wilcoxon signed-rank test. Statistical analysis was performed using JMP® Pro 14 (SAS Institute Inc., Cary, NC, USA) at a significance threshold of p<0.05 except for voxel-wise CBF analyses.
We explored which brain areas showed CBF changes after TAVI by conducting a voxel-wise comparison between CBF maps before and three months after TAVI at an exploratory significance threshold of p<0.005. CBF within the areas which changed after TAVI were calculated and were then used for a paired t-test between baseline and three months. A repeated measures linear mixed-model analysis was performed to evaluate changes in CBF and those in cognitive function tests. The details of the SPECT image pre-processing and analysis are shown in Supplementary Appendix 3.
Results
PATIENT CHARACTERISTICS
Clinical characteristics of the included and excluded patients are shown in Supplementary Table 1 and Supplementary Table 2. There were no significant differences in the results of cognitive function tests at baseline between the included and excluded patients. In the present study, the mean age was 83.2±4.5 years, and 80% were female. On the basis of a cut-off of <24 points for MMSE, five patients (33.3%) were considered cognitively impaired, whereas no patients were diagnosed as having dementia that required treatment with acetylcholine esterase inhibitors. No patients had luminal narrowing >25% in the carotid arteries, although we did not evaluate the status of the posterior artery.
PROCEDURAL CHARACTERISTICS AND CLINICAL OUTCOME
Procedural characteristics and clinical outcomes are shown in Table 1. No patients needed implantation of a second valve or showed myocardial infarction or cardiovascular death at 30 days after TAVI. Notably, no patients showed clinical symptoms or signs of transient ischaemic attack or stroke after TAVI. In addition, CO was also significantly increased at three months after TAVI compared with baseline (baseline, 4.03±0.88 vs 3 months, 5.10±1.14 L/min, p=0.0045).
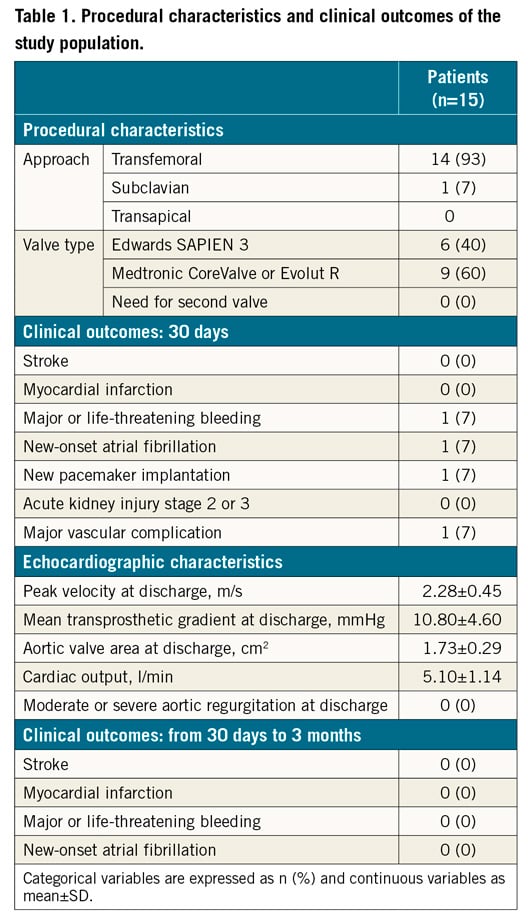
CHANGES IN COGNITIVE FUNCTIONS AFTER TAVI
At baseline, the mean scores of LM II, MMSE and GDS were 8.7±1.5, 24.6±1.3, and 4.3±1.1, respectively. LM II was significantly improved at three months after TAVI compared with baseline (baseline, 8.7±6.0 vs 3 months, 13.8±8.1, p<0.01). In contrast, there were no significant differences in MMSE or GDS during the study period (MMSE, baseline, 24.6±1.3 vs 3 months, 25.2±1.5, p=0.42; GDS, baseline, 4.3±1.1 vs 3 months, 4.2±0.9, p=1.0) (Figure 1). Among five patients (one third of the patients in the present study) with cognitive impairment at baseline, three showed that LM II was improved at three months after TAVI. In these three patients with MMSE scores 23, 18 and 13 at baseline, LM II scores increased at three months after TAVI from 6 to 12, 4 to 6, and 0 to 5, respectively.
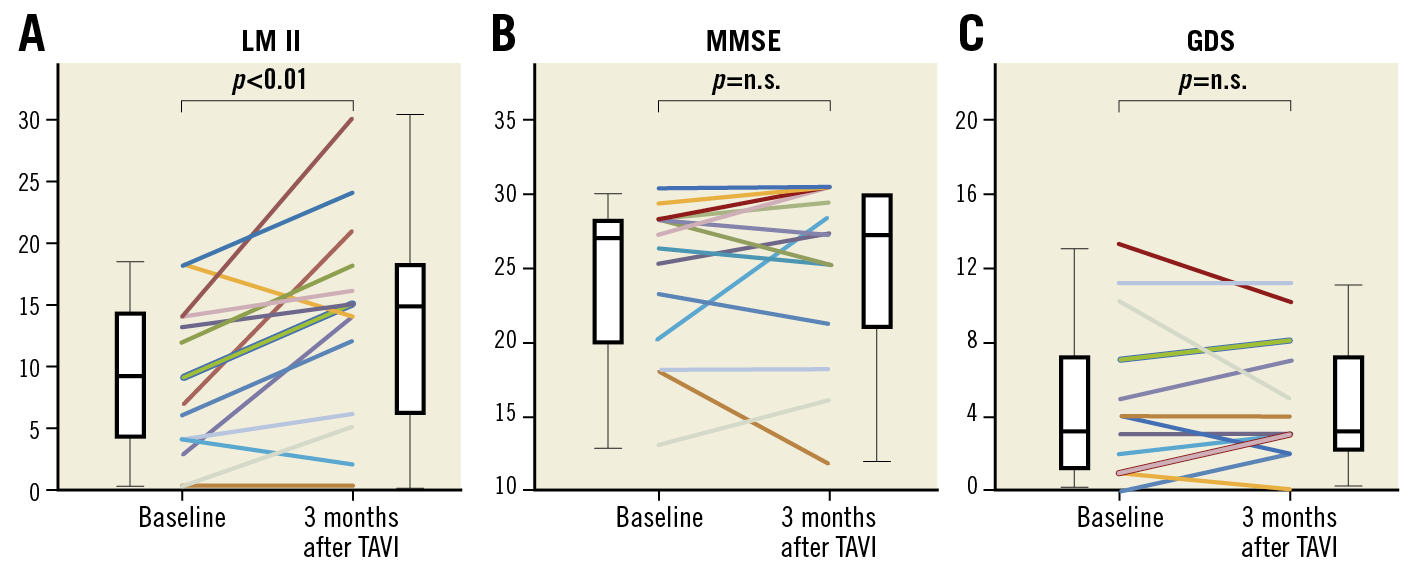
Figure 1. Changes in cognitive functions after TAVI in patients with severe AS. A) Logical Memory II (LM II) score was significantly improved at three months after transcatheter aortic valve implantation (TAVI) compared with baseline. B) & C) There were no significant differences in Mini-Mental State Examination (MMSE) or Geriatric Depression Scale (GDS) at three months after TAVI compared with baseline. n.s.: not significant
CHANGES IN CEREBRAL BLOOD FLOW AFTER TAVI
There were no significant differences in the whole CBF during the study period (baseline, 39.3±1.0 vs 3 months, 39.2±1.0 ml/100 g/min, p=0.76). However, CBF in specific regions was significantly increased after TAVI compared with baseline (baseline, 51.2±1.0 vs 3 months, 53.3±1.0 ml/100 g/min, p<0.001) (Figure 2A-Figure 2F). All five patients with cognitive impairment at baseline showed that CBF increased at three months after TAVI. Indeed, in these five patients with MMSE scores 23, 20, 18, 18 and 13 at baseline, CBF (ml/100 g/min) increased at three months after TAVI from 54.9 to 56.1, 48.3 to 50.6, 50.6 to 52.4, 53.6 to 56.3, and 51.0 to 53.3, respectively. This correlation between right hippocampal CBF and LM II scores was supported by the results from repeated measures linear mixed-model analysis (p=0.017) (Figure 2G). Moreover, the patients with increased CO after TAVI had significantly increased CBF in the right hippocampus compared with those without it (with increased CO, 1.06±0.07 vs without, 0.94±0.04, for changes in CBF in the right hippocampus after TAVI, p<0.01) (Figure 2H). Importantly, there was no significant difference in blood pressure during the study period (systolic blood pressure, baseline, 120.6±15.4 vs 3 months, 121.6±14.4, p=0.57; diastolic blood pressure, baseline, 62.9±12.4 vs 3 months 64.9±9.2, p=0.63).
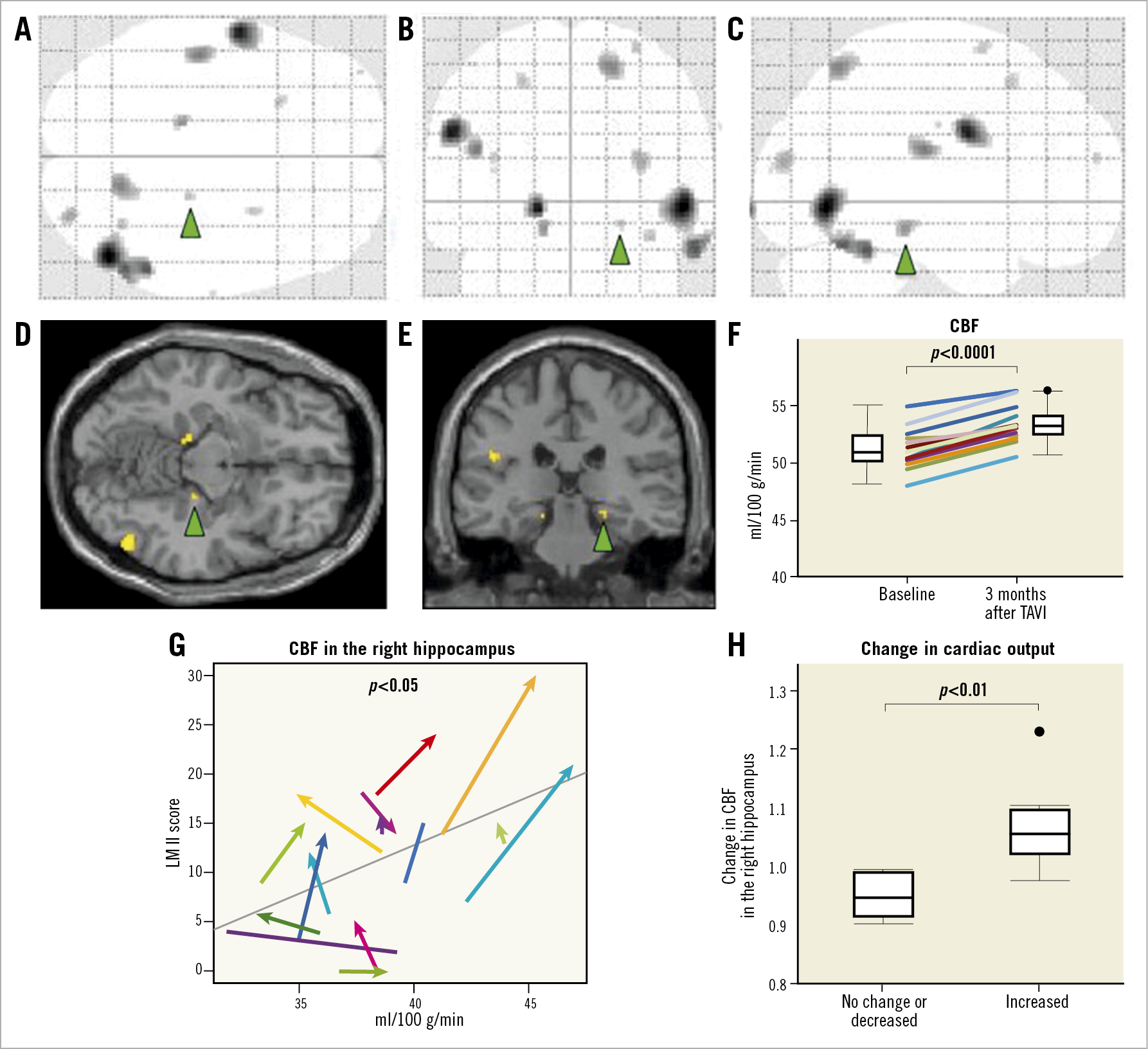
Figure 2. Changes in regional cerebral blood flow after TAVI and their associations with cognitive and cardiac functions. Glass brain representations showing TAVI-induced regional cerebral blood flow changes (black areas) from the coronal (A), axial (B), and sagittal (C) views (p<0.005 at each voxel). The coronal (D) and axial (E) slices including the right hippocampus are also presented. The green arrowheads indicate the right hippocampus. F) Local CBF was significantly increased after TAVI compared with baseline (baseline, 51.2±1.0 vs 3 months, 53.3±1.0 ml/100 g/min, W 60.0, p<0.0001). G) Linear mixed-effects model showed that CBF in the right hippocampus was positively correlated with LM II scores. H) The patients with increased cardiac output (CO) after TAVI had significantly increased CBF in the right hippocampus compared with those without it. CBF: cerebral blood flow; CO: cardiac output; LM II: Logical Memory II; TAVI: transcatheter aortic valve implantation
Discussion
The major findings of the present study were that 1) LM II was significantly improved after TAVI, 2) CBF in the local regions, including the right hippocampus, was significantly increased at three months after TAVI, 3) increase in CO was associated with that in CBF in the right hippocampus, and 4) CBF in the right hippocampus was positively correlated with LM II. To the best of our knowledge, this is the first study to demonstrate that TAVI may improve cognitive functions associated with increased cerebral perfusion especially in the hippocampus in elderly patients with severe AS.
CHANGES IN COGNITIVE FUNCTIONS AFTER TAVI
In the current practice guidelines, management of cognitive impairment needs to be improved, as proven therapeutic options are still lacking3. Although the number of patients with cognitive impairment and heart failure has been rapidly increasing in Western countries and Asia1,19, heart failure-associated cognitive impairment may be underestimated. Indeed, in the present study, five patients (33.3%) actually had cognitive impairment (MMSE <24). A recent study also demonstrated that 22~39% of patients with severe AS had impaired cognitive functions at baseline5,6. In the present study, although there were no significant differences in MMSE or GDS at three months after TAVI, LM II was significantly improved at three months after TAVI. Recent studies examined the global cognitive functions after TAVI, using MMSE and the Montreal Cognitive Assessment (MoCA)5,20,21. The MMSE, originally developed to screen for Alzheimer dementia, is currently widely used to assess post-stroke cognitive impairment22, although MMSE has been shown to lack sensitivity in the detection of very mild cognitive impairment22.
More recently, the MoCA has been developed to detect mild cognitive impairment with high sensitivity, which consists of seven cognitive domains, comprising orientation, attention, short-term memory, naming, visuospatial, language, and abstract reasoning5. LM II was developed specially to diagnose very mild cognitive impairment and episode memory12. In the present study, we used LM II instead of the MoCA for the following reasons. First, in a recent study, mean total MoCA score, especially short-term memory of the MoCA, was improved after TAVI5. Second, there was a significant improvement in the Immediate Recall Memory Test, with a trend towards an improved Delayed Recall Memory Test9. Third, LM II is a quantifiable neuropsychological test12. Taken together, it is possible that TAVI improves cognitive functions, especially LM II (episode memory), in the present study. In the present study, we had to exclude many patients, eventually analysing a relatively small number of patients, whose mean age was 83.2±4.5 years. A recent study has demonstrated that the risk and age of patients undergoing TAVI have become lower23. Thus, it remains to be elucidated whether TAVI improves cognitive function in younger patients with severe AS. Future studies with a large number of patients are needed to perform a multivariable analysis to adjust for possible factors contributing to the changes at follow-up.
ROLES OF INCREASED CEREBRAL BLOOD FLOW
Recent studies have shown that TAVI improves cognitive functions5,9,24. There were several hypotheses regarding the mechanisms of cognitive improvement after TAVI24,25,26. First, improvement of CBF due to improved CO after TAVI may contribute to the improvement of cognitive functions. Second, alleviation of physical symptoms and subsequent improvement in functional status may contribute to the improvement of cognitive functions. However, detailed mechanisms of the improvement of cognitive functions after TAVI remain to be examined.
Accelerated cognitive decline may result from chronic low cerebral perfusion in the long-term course of heart disease as a pathophysiological consequence between the heart and brain interactions3. In the present study, TAVI significantly improved CO, local CBF especially in the right hippocampus, and LM II scores. Importantly, CO was associated with CBF in the right hippocampus, with a positive correlation with LM II scores. Thus, we were able to elucidate that TAVI increases CO and cerebral perfusion (especially that in the hippocampus) associated with improved cognitive functions, probably through the heart-brain interaction in elderly patients with severe AS.
Notably, we have recently demonstrated that whole-brain low-intensity pulsed ultrasound therapy markedly ameliorates cognitive impairment associated with improved CBF in mouse models of dementia, in which endothelial nitric oxide synthase (eNOS) activation plays a central role27. It is conceivable that increased CBF caused by upregulated eNOS may also be involved in the beneficial effects of TAVI.
IMPORTANCE OF HIPPOCAMPUS FOR COGNITION
In the present study, although the whole CBF was not significantly increased, local CBF, especially that in the right hippocampus, was significantly increased after TAVI. Notably, we recently demonstrated that patients with chronic heart failure frequently have cognitive impairment, where the hippocampus blood flow is significantly decreased16. A possible mechanism of cognitive impairment in chronic heart failure is abnormality of the hippocampus, which is the important brain area for memory28. Moreover, the hippocampus is one of the brain regions most vulnerable to cerebral hypoxia29,30. Importantly, patients with obstructive sleep apnoea who underwent continuous positive airway pressure had improved cognitive function associated with improved grey matter volume in the hippocampus but not in the whole brain31. It is possible that the hippocampus is one of the watersheds and may be the first area where CBF reduction or improvement occurs. In the present study, although the whole CBF was not significantly increased, local CBF, including that in the hippocampus, was significantly increased after TAVI. Notably, in the present study, CBF in the local regions, not only in the right but also in the left hippocampus, was significantly increased after TAVI. The lack of statistical association between the left hippocampal blood flow and LM II scores may be due to the small sample size. Thus, it is possible that haemodynamic improvement by TAVI increases the perfusion in these regions, although the effect of cerebral hypoxia on brain abnormality in patients with severe AS remains to be elucidated.
Study limitations
Several limitations should be mentioned in relation to the present study. First, this study was a single-centre study with a relatively small number of patients. A fragility index value of 1 for our study indicated that an outcome change in a single patient would make the difference in the main outcome non-significant. Thus, future studies with a large number of patients are needed to perform a multivariable analysis to adjust for possible factors contributing to the changes at follow-up. Second, the present study focused on the abnormality of the hippocampus blood flow based on our previous study in rats30. However, substantial anatomical differences including the prefrontal cortex may exist between rats and humans. Third, there was a lack of control AS patients (without TAVI), although it is ethically and practically difficult to recruit such patients. Fourth, although we performed the commonly used tests for cognitive functions as previously reported5,9,12,13,14, we were unable to exclude a possible involvement of the learning effect. Future studies are needed to elucidate this effect. Fifth, the present study did not verify the cerebral structure and the CBF measurement using other modalities such as MRI. However, as mentioned above, we and others have already demonstrated that brain perfusion SPECT imaging is useful for solid assessment of quantitative cerebral perfusion and its relevance to cognitive impairment10,11.
Conclusions
In the present study, we were able to demonstrate for the first time that TAVI may improve cognitive functions associated with increased cerebral perfusion especially in the hippocampus in elderly patients with severe AS.
|
Impact on daily practice Recent studies suggest that cognitive decline may result from chronic low cerebral perfusion in the long-term course of heart disease as a pathophysiological consequence between the heart and brain interactions. Based on the present study, TAVI may improve cognitive functions associated with increased cerebral perfusion especially in the hippocampus in elderly patients with severe AS. |
Acknowledgements
We thank Mari Ohtsuki, a clinical psychologist at Tohoku University Hospital, for assessment of cognitive function in the present study.
Funding
This work was supported in part by the Grants-in-Aid for the Scientific Research and the Grants-in-Aid from Mitsui Sumitomo Insurance Welfare Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

