
The prevalence of aortic stenosis (AS) and cognitive impairment increases with age1,2. In the context of a rapidly ageing population3, the number of individuals living with severe AS and dementia is expected to increase worldwide. Cognitive decline is indeed common among individuals with AS4,5. Whether this is due to decreased cardiac output (CO)6, shared risk factors for atherosclerosis7, or a more complex “cardiocerebral continuum”, deserves further discussion.
The uptake of transcatheter aortic valve implantation (TAVI) is rapidly and continuously increasing8. Despite the known efficacy of TAVI on hard outcomes such as death, and stroke9, the association with other patient-important outcomes such as cognitive impairment remains unknown. Understanding the association between TAVI and longitudinal changes in cognition is important, and therefore also the inherent age-associated risk of incident cognitive decline and dementia2.
In this issue of EuroIntervention, Tsuchiya et al10 report the results of a small observational study evaluating changes in cognitive function, CO, and cerebral blood flow (CBF) before and after TAVI.
The study included 15 elderly patients (mean age 83.2±4.5 years) with severe AS. They used the Logical Memory II (LM-II) test and the Mini-Mental State Examination (MMSE) in order to ascertain cognitive changes at three months after TAVI relative to baseline testing performed before the procedure. The Geriatric Depression Scale (GDS) was used to assess depression. They quantified CO by echocardiography and CBF by means of single-photon emission computed tomography (SPECT) at the same time points as cognitive assessment. This study has three main findings. First, while mean LM-II was higher at three months after TAVI relative to its baseline assessment (8.7±6.0 vs 13.8±8.1, p<0.01), there were no differences in mean MMSE or GDS scores. Second, despite no differences in overall CBF before and after TAVI, there was an increase in right hippocampal CBF from 51.2±1.0 to 53.3±1.0 ml/100 g/min (p<0.001) at three months after TAVI. All patients with MMSE <24 showed increases in CBF. Third, although there were no differences in CO before and after TAVI in the whole cohort, the group of patients with increased CO after TAVI showed increased CBF within the right hypothalamus.
The study of Tsuchiya et al10 is innovative from the perspective of concurrently evaluating changes in cognitive performance, CO, and CBF after TAVI. However, given the limitations of this study, its results should be interpreted with caution and considered as hypothesis-generating; thus, future studies for further validation are required. Of note, the design and combination of statistical tests are underpowered for detecting hypothetical effect sizes in cognition, CO, and CBF after TAVI. Second, none of the analyses was adjusted for confounders with the potential to alter outcomes such as educational level and depression for cognition, blood pressure for CBF, and history of coronary artery disease and left ventricular ejection fraction for CO. Third, this is not a randomised controlled trial; hence, actual or potential causal associations between improvements in cognitive functioning and increases in CO and CBF after TAVI remain unknown. Fourth, assessing cognition is challenging, since results may differ across cognitive batteries11. More specifically, the LM-II is a measure of delayed recall and is therefore more sensitive for detecting cognitive impairment in patients with degenerative dementias (e.g., Alzheimer’s disease), rather than for vascular types, in which executive functions are more frequently impaired. Similarly, the MMSE has poor sensitivity for detecting executive and attentional deficits typical of dementias with a vascular component12,13. Another specific and important problem of the LM-II is that it is very susceptible to learning effect14. Finally, depression is a treatable cause of poor cognitive performance and a usual confounder when testing cognitive functioning15. While the authors used the GDS for assessing depression, it is not clear how many patients with MMSE <24 had low scores because of being depressed.
THE CARDIOCEREBRAL CONTINUUM
Severe AS is associated with increased arterial stiffness4,16. This plays a major role on the cerebral vascular system, and it prompts microvascular ischaemia, structural changes and remodelling, leading to vascular ageing and subsequent cognitive impairment12,17,18,19. Neurocognitive decline in individuals with AS4,5 further highlights the cardiocerebral linkage. It is important to point out that cognitive tests differ substantially across TAVI studies20. Moreover, Lazar and colleagues5 showed that a significant proportion of older patients undergoing TAVI had impaired cognitive function before the procedure. A recent meta-analysis21 showed no differences in cognitive outcomes before and after TAVI. Interestingly, there was only an initial improvement at 30 days; however, there were no differences at 3, 6 and 12 to 34 months after TAVI21. Notably, the lack of individual patient data in the meta-analysis precludes the assessment of pre-existing cognition and functional status; hence, the unchanged cognitive status after TAVI may not necessarily imply stability at an individual patient level.
While TAVI may be associated with improved CBF, it has also been associated with silent ischaemic lesions in two thirds of the cases20. Importantly, studies before the TAVI era have shown that silent brain infarcts are strongly associated with cognitive impairment and dementia22,23; thus, TAVI could be regarded as a potential cause for worsening pre-existing conditions, although this is still under debate24. In this regard, cerebral embolic protection devices may have an important role25,26. Nonetheless, the Neurologic Academic Research Consortium (NeuroARC) agrees with the lack of a conclusive association between acute procedure-related subclinical brain lesions and long-term neurological or cognitive outcomes27. In addition, the detection of neurological or cognitive sequelae depends on the nature, sensitivity, and timing of assessments27. Proposed underlying mechanisms of the “cardiocerebral continuum” potentially affecting cognitive outcomes in patients undergoing TAVI are shown in Figure 1.
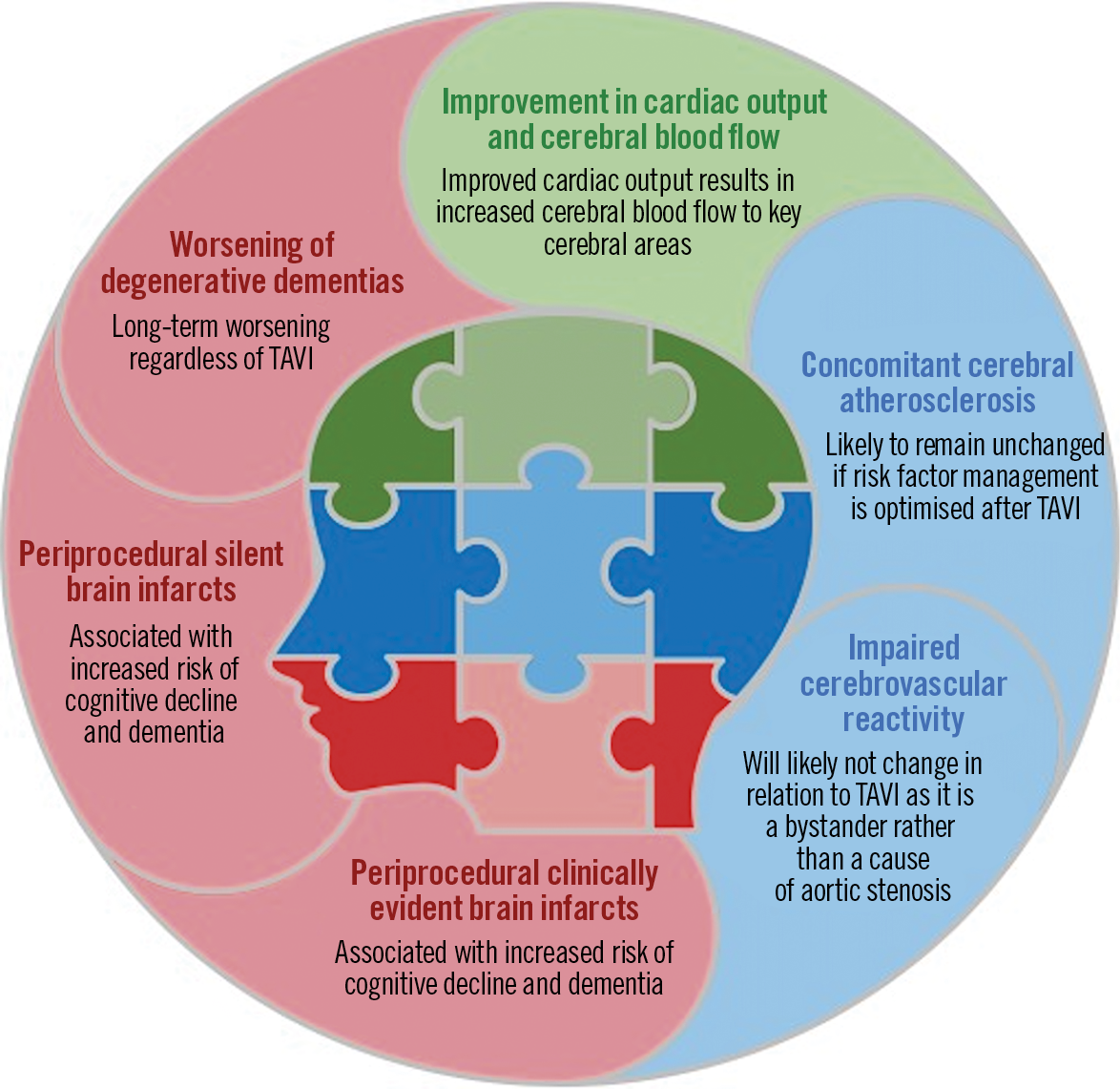
Figure 1. Cardiocerebral continuum in cognitive function after transcatheter aortic valve implantation (TAVI).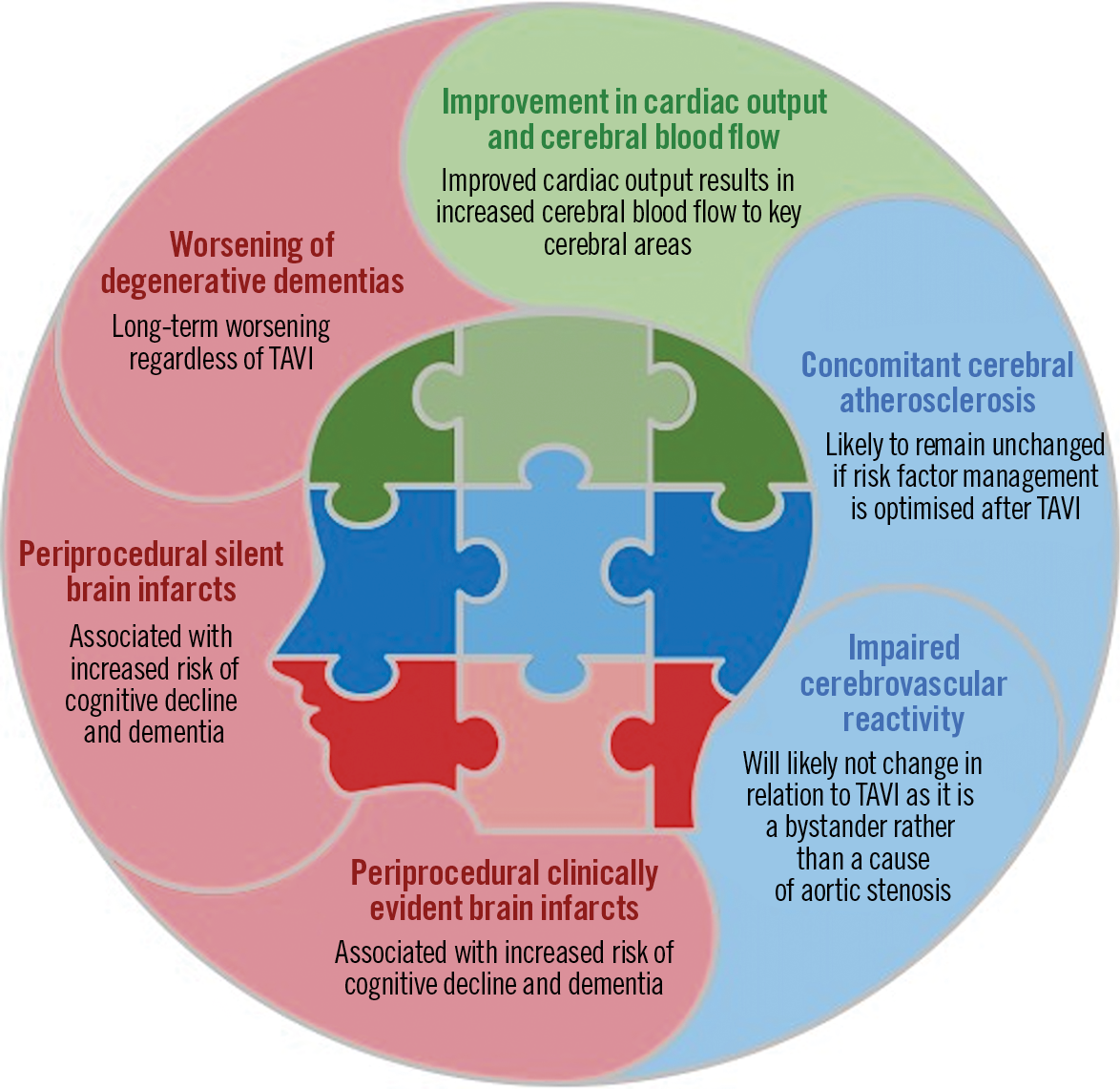
In summary, whether cognitive function is affected by TAVI remains unknown; certainly, a systematic neurological assessment may help to elucidate this question. Randomised clinical trials including the whole range of preprocedural risks and ages would be necessary to address this important knowledge gap.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

