Abstract
Aims: To evaluate the impact of serological, imaging and clinical measures of cerebral injury on patient self-sufficiency and survival after transcatheter aortic valve implantation (TAVI).
Methods and results: Before and three days after TAVI, neuron-specific enolase (NSE), cerebral diffusion-weighted magnetic resonance imaging (DW-MRI) and neurological performance utilising National Institutes of Health Stroke Scale (NIHSS) were assessed. Self-sufficiency was determined with established score systems (instrumental activities of daily living score, Barthel Index). Parameters of cerebral injury were investigated for their impact on self-sufficiency and all-cause mortality after 30 days and one year. Sixty-one patients were enrolled (logistic EuroSCORE: 26.4±18.1, STS score: 7.9±5.7), of whom 39 completed the imaging protocol. The incidences of NSE increase, new embolic events in DW-MRI, and neurological deficit early after TAVI were 52.4%, 71.8% and 6.6%, respectively. The degree of concomitant comorbidities, reflected by higher risk scores, had significant impact on outcome. Plasma levels of NSE and new emboli in DW-MRI were neither related to self-sufficiency nor to survival one year after TAVI.
Conclusions: In this observational pilot study, “silent” cerebral injury is neither related to dependent lifestyle nor to mortality during the first year after TAVI. However, long-term follow-up is needed to elucidate fully the impact of silent stroke. Clinical trials number: NCT00883285.
Introduction
Transcatheter aortic valve implantation (TAVI) has become a valid therapeutic approach for patients with symptomatic aortic stenosis at high or excessive surgical risk1,2. Peri-interventional stroke is a challenging complication of TAVI, known to be associated with disability and adverse outcome3. Procedure-related silent cerebral embolic events are frequently observed with diffusion-weighted magnetic resonance imaging (DW-MRI)4-6. In view of the considerable increase of life expectancy, the potential expansion of TAVI to patients at lower surgical risk, and the availability of cerebral embolic protection devices, the mid-term implications of (sub)clinical brain injury related to TAVI are essential for patients, interventional cardiologists, and cardiac surgeons7.
Serological and imaging parameters of cerebral injury have frequently been used as surrogate parameters of peri-interventional stroke risk and further offered information on the incidence, localisation and size of embolic events8. Interestingly, various established interventional and surgical cardiovascular procedures encompassing left-heart catheterisation, carotid intervention and endarterectomy, coronary artery bypass grafting, and surgical valve repair are associated with cerebral injury in DW-MRI8-10. The incidence of “silent” peri-interventional cerebral embolic events ranges from 4% after pulmonary vein isolation to up to 84% after TAVI5,11. In comparison, the incidence of embolic events after surgical aortic valve replacement in patients at lower surgical risk was 38%12. Silent embolic events are of interest since large-scale imaging studies after carotid intervention and stroke demonstrated a correlation of embolic burden and neurological outcome8,13. Clinically, the National Institutes of Health Stroke Scale (NIHSS) is the method of choice to investigate the severity of apparent stroke. Here, we prospectively investigated the impact of established serological, imaging and clinical measures of cerebral injury on functional outcome and survival to determine the prognostic value of silent and apparent cerebral embolic events up to one year after TAVI.
Methods
From November 2008 to March 2010, patients scheduled for TAVI were screened for participation in the trial. Indication for TAVI was in concordance with the consensus statement14. The study protocol was approved by the local institutional review board and followed the Declaration of Helsinki guidelines. Written informed consent was obtained from all patients.
INCLUSION CRITERIA
Major study inclusion criteria were: a) severe, symptomatic aortic stenosis with or without regurgitation and high or excessive perioperative risk, b) echocardiographic aortic valve annulus diameter >20 and <27 mm, and c) diameter of the ascending aorta <45 mm. Exclusion criteria included contraindications to DW-MRI, e.g., permanent pacemaker implantation, claustrophobia, or haemodynamic instability impeding transport to DW-MRI, hypersensitivity or contraindication to post-interventional dual platelet inhibition; sepsis or active endocarditis; bleeding diathesis or coagulopathy; recent cerebrovascular accident; mitral or tricuspid valvular insufficiency (> grade II); left ventricular or atrial thrombus; previous aortic valve replacement; progressive disease with life expectancy <1 year, and inability to give written informed consent. Furthermore, patients without a self-sufficient lifestyle or with a psychiatric disorder were excluded.
STUDY DESIGN AND OUTCOME MEASURES
Baseline exams were performed prior to TAVI. After careful investigation of past medical history, the mortality risk was estimated by both the logistic EuroSCORE and the risk score of the Society of Thoracic Surgeons (STS mortality risk score)14,15. The risk of permanent stroke was approximated (STS permanent stroke score). Clinical examinations according to the NIHSS were performed. A 12-lead surface electrocardiogram, serological and haematological analyses, transthoracic and transoesophageal echocardiography, colour-coded duplex sonography of the extracranial carotid arteries, ventriculography, and coronary angiography were obtained in all patients. Preprocedural cerebral DW-MRI was performed the week before TAVI. First post-procedural investigations were scheduled three days after TAVI. Follow-up investigations were performed 12 months after TAVI (Figure 1).
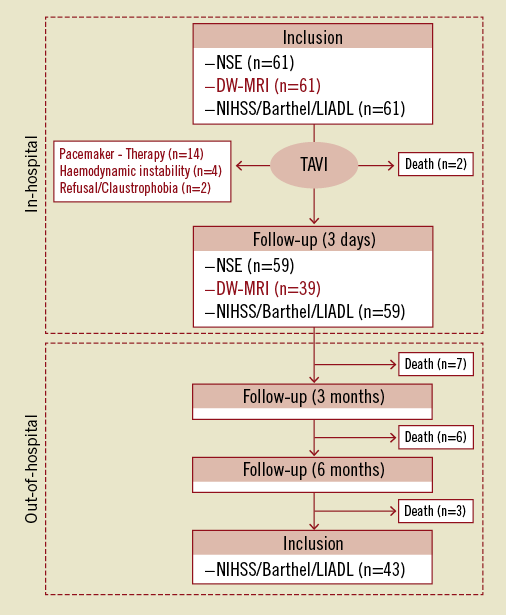
Figure 1. Study protocol. Sixty-one patients were eligible for the study protocol and represent the intention-to-treat cohort. Serological and clinical parameters of cerebral injury were investigated in all patients. Twenty-two patients could not be followed up with diffusion-weighted magnetic resonance imaging (DW-MRI) due to post-interventional pacemaker implantation, new onset of claustrophobia/refusal or haemodynamic instability. Thirty-nine patients formed the per-protocol dataset and were serially investigated with respect to serological, imaging and clinical parameters of TAVI-related cerebral injury. LIADL: Lawton’s instrumental activities of daily living scale; Barthel: Barthel Index; NIHSS: National Institutes of Health Stroke Scale; NSE: neuron-specific enolase
SEROLOGICAL, IMAGING AND CLINICAL PARAMETERS OF CEREBRAL INJURY
As a serological marker of brain injury, neuron-specific enolase (NSE) levels were utilised4,16. NSE was quantified in venous blood samples with a commercially available enzyme immunoassay following the manufacturer’s instructions (Liaison® NSE; DiaSorin, Saluggia, Italy).
Imaging parameters encompassed embolic events in cranial DW-MRI4-6. Any new embolic lesion in DW-MRI after TAVI was considered as cerebral injury. Patients with procedure-related contraindications to MRI (e.g., necessity for a permanent pacemaker) would have undergone computed tomography in case of new onset of focal neurological deficit.
Clinical investigations consisted of standardised assessment of neurological status utilising the NIHSS3. Any change of NIHSS was considered as significant. Transient ischaemic attack (TIA) was defined as a focal neurological event that was entirely reversible within <24 hours in the absence of any new imaging findings. A minor stroke was defined as an event associated with a modified Rankin Scale (mRS) score ≤1. Major stroke was defined by a score ≥2 at 30 days after the index procedure3,13.
ENDPOINTS
To assess clinical consequences of cerebral injury after TAVI, the population was evaluated for self-sufficiency in daily activities at one year as a primary endpoint. For the assessment of self-sufficient lifestyle a combination of established scoring systems (Barthel Index ≥85 and Lawton’s instrumental activities of daily living (LIADL) scale ≥52) was utilised17,18. Patients meeting both criteria were categorised as self-sufficient, requiring only minimal or no assistance with daily activities19. The prospective evaluation of self-sufficiency in daily living is critical, since living at home, in a residence or nursing facility is not selective and can be associated with both self-sufficiency and dependence. Mortality was obtained during follow-up visits at three and 30 days, three, six and 12 months, and every six months thereafter. Further information, e.g., the cause of death, was obtained from the treating hospital or general practitioner charts. Established clinical endpoints were obtained in accordance with the VARC criteria3.
CRANIAL MRI AND IMAGING ANALYSIS
Cranial MRI was performed using a 1.5 Tesla whole body system (Intera; Philips Medical Systems, Best, The Netherlands). Quantitative DW-MRI was performed before and within four days after TAVI. The imaging protocol included transversal and coronal DWI, transversal T2-weighted turbo spin-echo (turbo spin-echo (TSE); repetition time (TR)/echo time (TE): 4800/100 ms) and fluid attenuated inversion recovery (FLAIR; TR/TE 6000/120 ms) sequences. DWI was performed with a spin-echo echo-planar pulse sequence (TE: 78 ms; TR: 2,921 ms; echo-planar imaging factor: 77; field of view: 240 mm; matrix: 128×256; section thickness: 5 mm; intersection gap: 1 mm; total acquisition time: 21.4 seconds) with diffusion sensitisation b-values of 0, 500 and 1,000 s/mm². Apparent diffusion coefficient maps were obtained in all cases.
Pre-existing brain abnormalities (e.g., microangiopathy, infarctions, or atrophy) and the appearance of new hyperintense lesions in DWI on postoperative scans were evaluated. Only diffusion abnormalities consistent with embolic lesions were included in the analysis. Diffuse alterations in the diffusion-weighted image or patterns of watershed ischaemia were excluded. Likewise, post-interventional new lesions were determined on the DWI images with maximum contrast between lesion and normal tissue signal. Scans were read by two experienced radiologists without knowledge of the timing of the imaging with respect to therapy and blinded to the clinical and neurological status of the patient. In case of discrepancy, a consensus reading was held. For image analysis, the commercially available software of the MRI unit was used (Viewforum; Philips Medical Systems, Best, The Netherlands).
TRANSFEMORAL AORTIC VALVE IMPLANTATION (TAVI)
The third-generation (18 Fr) CoreValve® revalving system (Medtronic Inc., Minneapolis, MN, USA, previously CoreValve Inc., Irvine, CA, USA) consists of a trileaflet bioprosthetic porcine pericardial tissue valve mounted and sutured in a self-expanding nitinol frame. Details of TAVI have been published previously20,21. In the present study only third-generation (18 Fr) devices were used. TAVI was performed with local anaesthesia in combination with a mild systemic sedative treatment. During TAVI, the patients received 500 mg of acetylsalicylic acid (ASS) and weight-adjusted intravenous heparin to achieve an activated clotting time of 300 to 350 sec for the duration of the procedure. A loading dose of 300 mg clopidogrel hydrogen sulphate was administered the day before TAVI. Dual antiplatelet treatment was continued with 100 mg of ASS and 75 mg of clopidogrel for six months followed by ASS monotherapy.
ECHOCARDIOGRAPHY, DUPLEX SONOGRAPHY, CORONARY ANGIOGRAPHY
All echocardiographic studies were performed with commercially available equipment (iE33; Philips Medical Systems, Best, The Netherlands). Left ventricular function was measured with transthoracic echocardiography according to the recommendations of the American Society of Echocardiography. Sources of embolism were investigated by means of transoesophageal echocardiography. All patients underwent sonographic examination for detection of atherosclerotic lesions of the carotid arteries. Cardiac catheterisations and standard coronary angiography were performed with 6 Fr catheters.
STATISTICAL ANALYSES
Continuous variables are presented as mean ± standard deviation if normally distributed and as median (interquartile range) if not normally distributed. Categorical variables are given as frequencies and percentages and were compared by χ² (chi-square) statistics or Fisher’s exact test. Continuous variables were tested for differences by means of a two-sided, unpaired Student’s t-test for comparison between groups and with a two-sided, paired Student’s t-test for intragroup comparison. The association between continuous variables was examined by use of Spearman’s rank correlation. The clinical endpoints were plotted in relation to clinical, serological and imaging parameters of cerebral injury by the Kaplan-Meier method and were compared with the log-rank test. A value of p<0.05 was considered statistically significant. All analyses were conducted with IBM SPSS Statistics version 20.0.0 (IBM Corporation, Somers, NY, USA).
Results
PATIENT POPULATION
A total of 61 patients constituted the study population. Median follow-up time was 320 days (interquartile range: 270-410 days), and the follow-up rate was 100%. Baseline characteristics were within the range of previous TAVI studies1,2,4-6. The study cohort had a mean logistic EuroSCORE of 26.4±18.1 and a mean STS score of 7.9±5.7. Further details are depicted in Table 1. Serological and clinical parameters of cerebral injury were obtained in all patients. Imaging parameters were obtained at 3±1 day after TAVI. DW-MRI after TAVI could not be obtained in 22 patients (36.1%) due to death (n=2, 3.3%), post-interventional permanent pacemaker implantation (n=14, 22.9%), claustrophobia/refusal (n=2, 3.3%), or haemodynamic instability (n=4, 6.6%). The other 39 patients (64.9%) completed the imaging protocol (Figure 1).
SEROLOGICAL PARAMETERS OF CEREBRAL INJURY
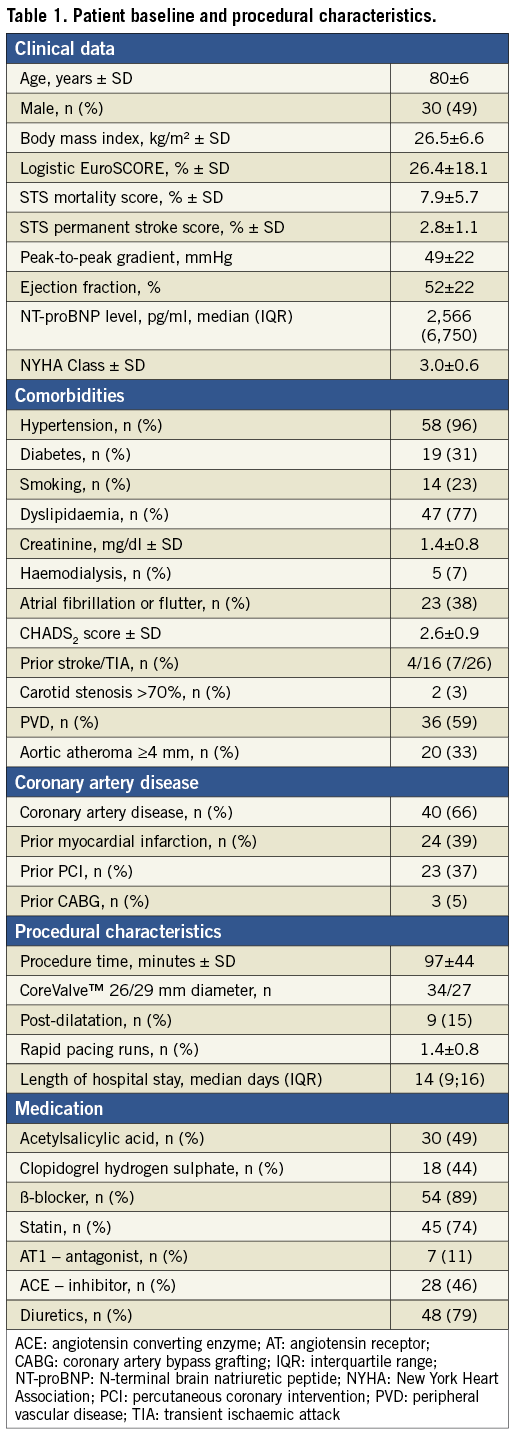
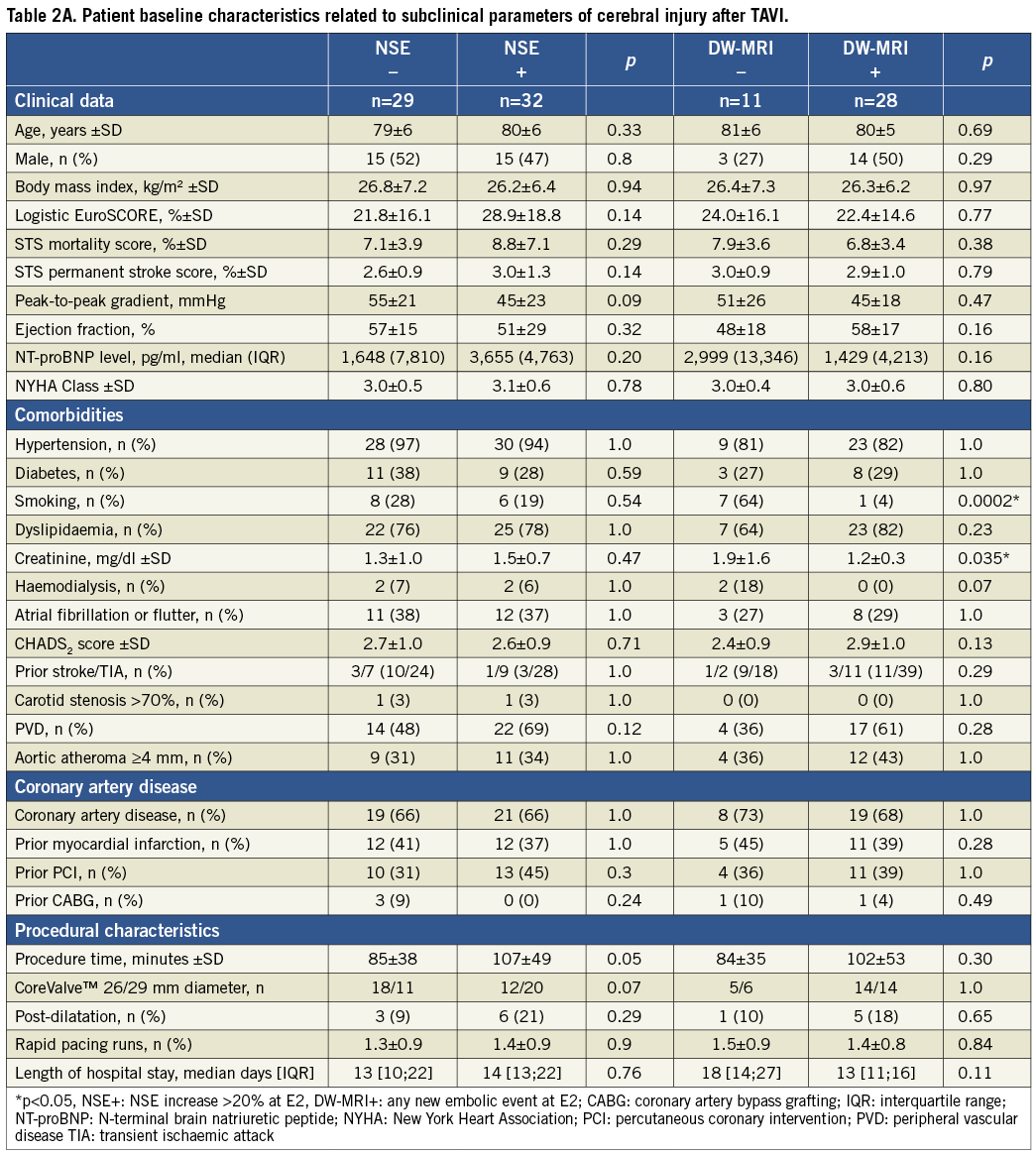
Twenty-nine patients (47.5%) had a significant increase of NSE early after TAVI. After analyses of receiver operator characteristics, an NSE increase of ≥20% was considered as significant. To investigate potential sources of post-interventional increase of NSE, baseline characteristics of patients with and without serological measures of cerebral injury were compared. No potential predictors of significant NSE increase were detected as given in Table 2A.
IMAGING PARAMETERS OF CEREBRAL INJURY
All 61 patients showed brain atrophy and hyperintense white matter lesions at baseline. Lacunar defects were obtained in 25 patients (40.1%), and six patients showed old territorial infarcts (9.8%). No patient suffered from acute ischaemic lesions in the baseline DW-MRI.
On the follow-up MRI, a total of 134 new lesions were detected (range: 0-19 lesions per patient) in 28 of 39 patients (71.8%). 65 of 134 lesions (48.5%) were present in patients with lacunar defects and old infarcts. Concerning new ischaemic lesions, 112 were observed in supratentorial and 22 in the infratentorial position, respectively. Most supratentorial lesions (68.8%) were located on the left side. New lesions were equally distributed in number and localisation. Most lesions were found in the territories of the middle cerebral arteries (MCA, n=55), followed by the posterior cerebral artery (PCA, n=34), anterior cerebral arteries (ACA, n=23) and vertebrobasilar arteries (n=22).
To elucidate potential sources of TAVI-related embolic events in DW-MRI, baseline characteristics of patients with and without new events were compared in Table 2A.
CLINICAL PARAMETERS OF CEREBRAL INJURY
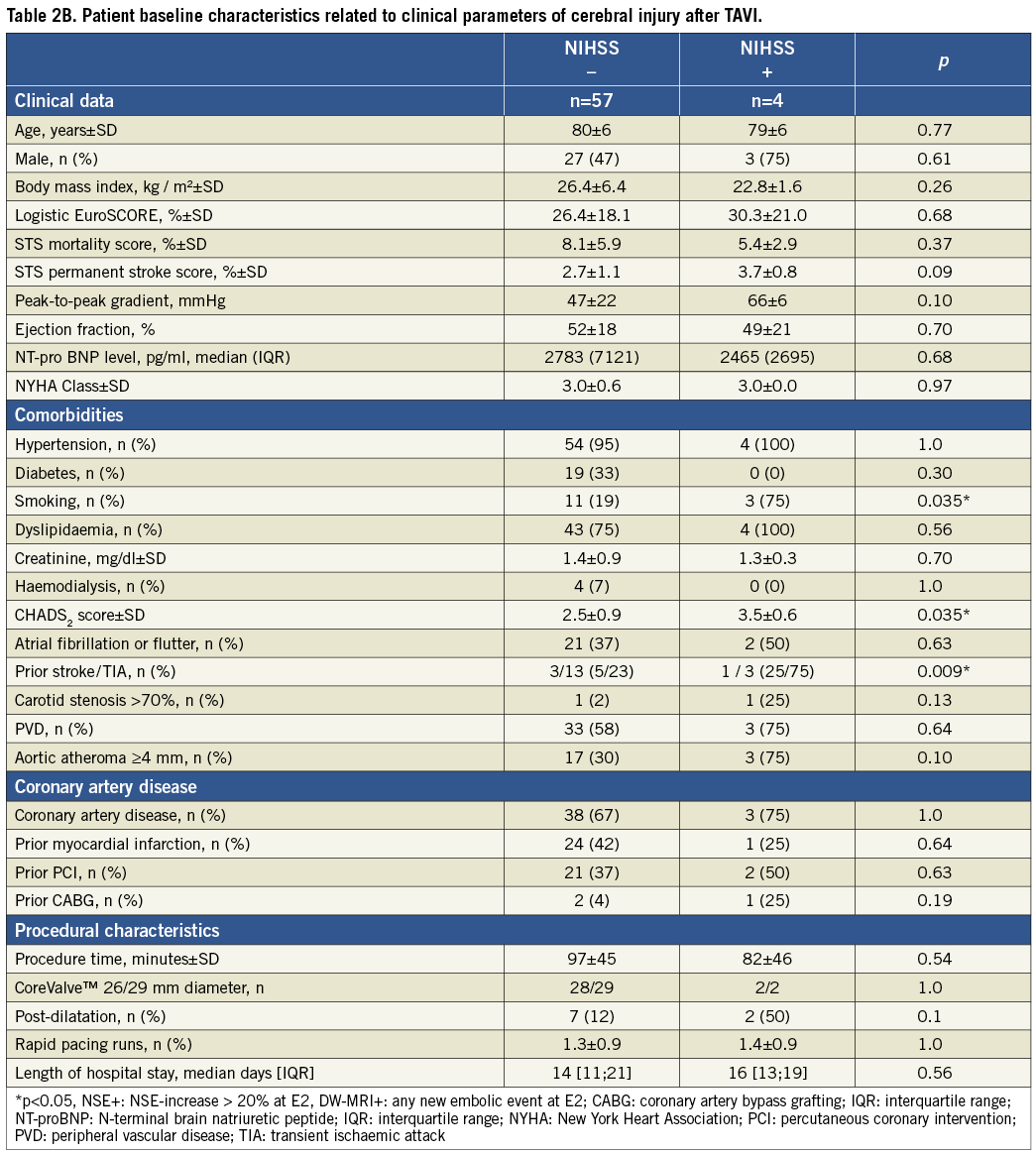
Four of 61 patients (6.6%) met the VARC criteria of peri-interventional stroke3. All 22 patients who could not undergo the post-procedural DW-MRI revealed no neurological deficit (NIHSS: 0) at any time. To identify potential risk factors of TAVI-related focal deficit (NIHSS >0), the baseline characteristics were dichotomised and compared. Patients without post-interventional focal neurological deficit during the first year after TAVI revealed a significantly lower rate of previous cerebrovascular events, a lower CHADS2 score, and were significantly more often never smokers (Table 2B).
SURVIVAL
All patients were followed up for the incidence of major adverse cardiac and cerebrovascular events (Table 3). In this cohort, the survival after 30 days and one year was 87% and 71%, respectively. Patient population was dichotomised for Kaplan-Maier analyses with respect to serological, imaging and clinical measures of cerebral injury. Neither NSE increase nor new lesions in DW-MRI were predictive for all-cause mortality one year after TAVI (Figure 2). Two patients suffered from comprehensive neurological deficits (NIHSS 16 and 14), this becoming clinically apparent within 48 hours after TAVI. Both patients with major strokes demonstrated comprehensive residual deficits throughout the follow-up and died on days 69 and 184 after TAVI, respectively (Figure 2).
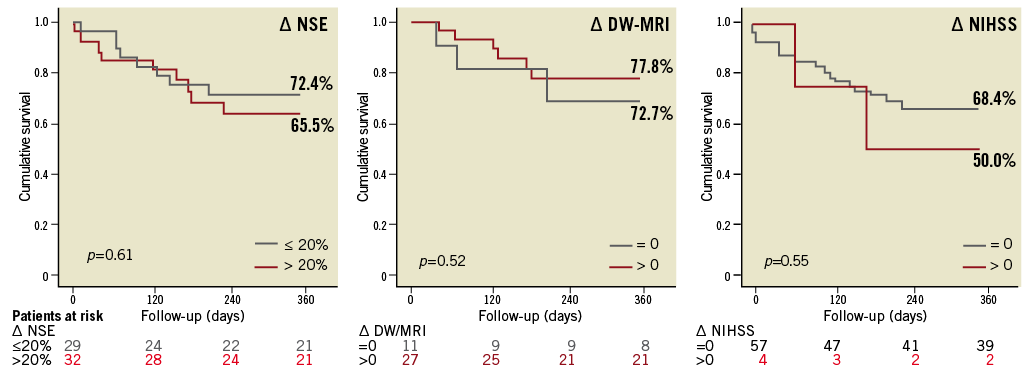
Figure 2. Time-to-event curves for all-cause mortality. Serological, imaging and clinical parameters of cerebral injury were related to survival after TAVI by means of the Kaplan-Meier method. Delta (∆) indicates the differences of pre- and early post-interventional NSE serum levels (∆ NSE) and the presence of any new embolic cerebral lesion in DW-MRI (∆ DW-MRI) and focal neurological performance (∆ NIHSS). None was predictive for all-cause mortality. However, with respect to NIHSS-positive patients, the statistics are based on a low number of events (n=4).
LIFESTYLE
The incidence of sustained self-sufficient lifestyle throughout the first year after TAVI was 75.4% (46 of 61 patients). Univariate analyses of baseline characteristics were performed to identify predictors of self-sufficient lifestyle one year after TAVI. Patients depending on significant help in daily activities at any time during the first post-interventional year demonstrated a significantly higher burden of concomitant disease, reflected in significantly higher risk scores and BNP values at baseline (Table 4). The one-year survival of the dependent patient subgroup was 7% (1 of 15 patients), whereas the survival of patients remaining self-sufficient was 89.1% (41 of 46 patients; p<0.001). Concomitantly, the length of hospitalisation was significantly higher in patients with dependent lifestyle after TAVI (Table 4). Pre-interventional status was comparable in both subgroups, reflected in similar Barthel scores (self-sufficient: 95.2±9.5, dependent: 91.1±13.2; p=0.33). Neither the significant elevation of the biomarker NSE nor new embolic events in DW-MRI was predictive for self-sufficient lifestyle after TAVI (Figure 3). In this cohort, four clinically apparent events were obtained, precluding valid statistical assumptions on prognostic impact. More specifically, both patients with major strokes (NIHSS: 14 [mRS: 4] and 16 [mRS: 5]) demonstrated impaired functional status and dependent lifestyle at some time after TAVI and finally died. The two patients with minor strokes (NIHSS: 1 and 4) demonstrated no residual deficits at discharge and follow-up (mRS: 0). Both patients remained completely independent in daily living activities after the hospital stay and revealed no residual or recurrent neurological deficits during the follow-up period.
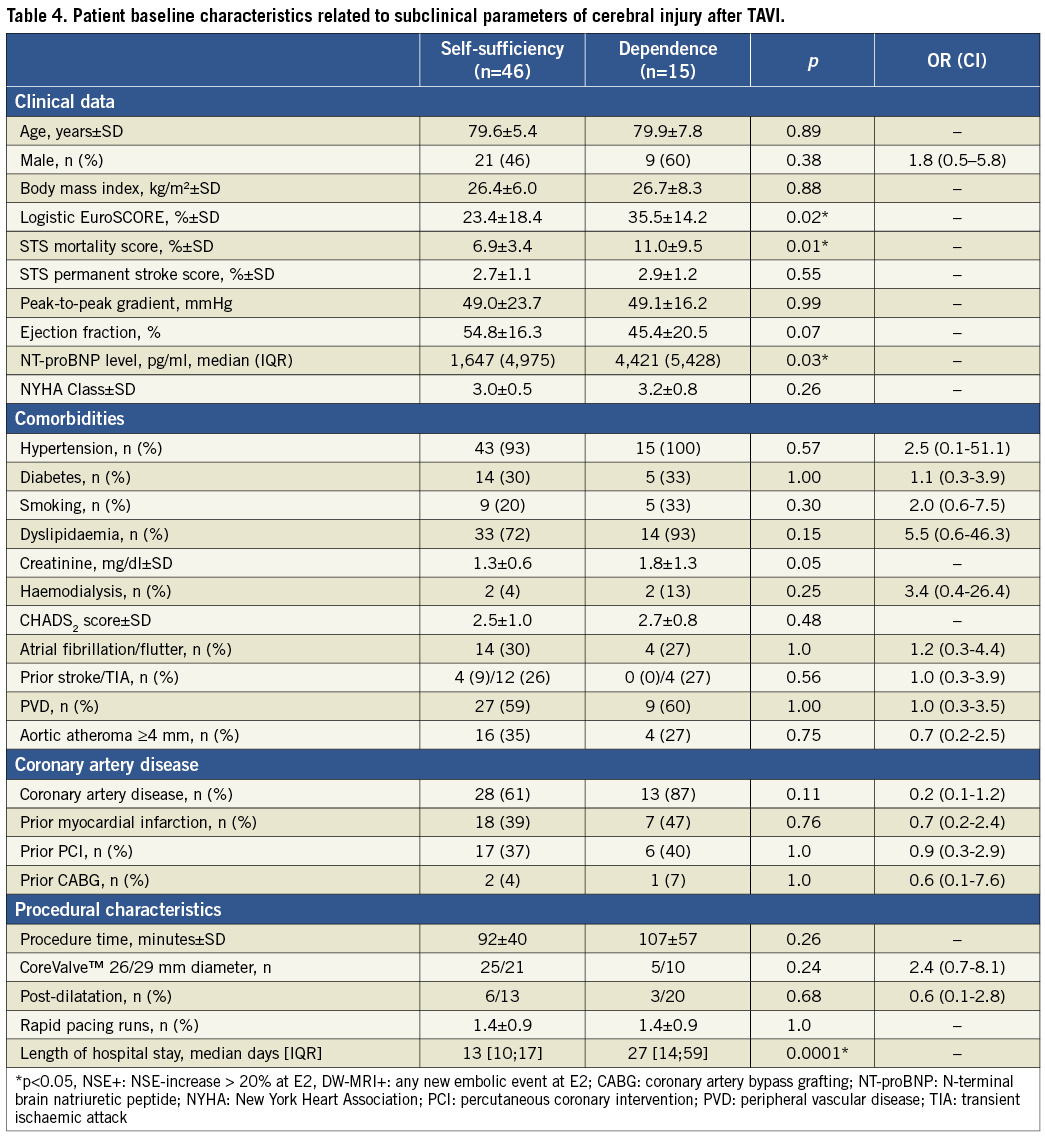
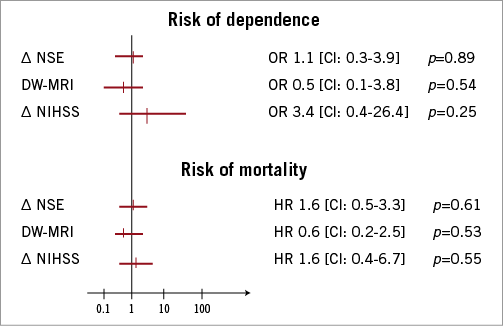
Figure 3. Relative risk of dependent lifestyle and mortality after TAVI. Serological, imaging and clinical measures of cerebral injury were related to lifestyle and survival after TAVI by means of univariate analysis. Delta (∆) indicates the differences of pre- and early post-interventional NSE serum levels (∆ NSE), the presence of any new embolic cerebral lesion in DW-MRI (∆ DW-MRI) and focal neurological performance (∆ NIHSS).
Discussion
TAVI has evolved to be a valid therapeutic option in patients with severe symptomatic aortic stenosis at high surgical risk. However, peri-interventional stroke is a shortcoming of the procedure with apparent and silent cerebral embolic event rates as high as 3-10% and 73-84%, respectively4-6. Symptoms and clinical manifestations of cerebral injury are highly variable, ranging from transient loss of orientation to persistent post-interventional disability22. Taking the procedure’s potential expansion into account and also the lack of information regarding the prognostic value of the frequent, procedure-related cerebral embolic events, this is the first study to demonstrate that silent measures of cerebral injury have no detrimental influence on patients’ self-sufficiency and survival for up to 12 months after TAVI.
MEASURES OF CEREBRAL INJURY - SEROLOGICAL
In our study, NSE was predictive neither for patient self-sufficiency nor for survival one year after TAVI. This surrogate parameter was chosen since serological investigations in patients with embolic stroke or hypoxaemia identified NSE as a valid biomarker of cerebral injury. NSE serum concentration has been found to correlate closely with the volume of ischaemic tissue early after embolic stroke16. Furthermore, NSE has a high negative predictive value for hypoxaemic cerebral injury three days after cardiopulmonary arrest23. Ultimately, NSE was correlated with neurocognitive decline and embolic events in DW-MRI in a single-centre observation12. However, most studies and a recent meta-analysis obtained no association of NSE and neurocognitive decline or adverse outcome following cardiac surgery24. As in our initial experience, NSE levels demonstrated no correlation with embolic burden in DW-MRI following TAVI4. Haemodilution and transfusion are known confounders, since NSE is set free from haemolysed erythrocytes and is not strictly neuron-specific12. Our results question the usefulness of NSE as a surrogate marker for neuroprotective approaches in TAVI trials.
MEASURES OF CEREBRAL INJURY - IMAGING
We were able to demonstrate that the occurrence of silent embolic events in DW-MRI had no negative impact on lifestyle and survival one year after TAVI. DW-MRI is the gold standard for the detection, localisation and quantification of acute embolic cerebral events and was previously utilised to investigate the incidence of cerebral embolic events after TAVI4-6. “Silent” cerebral embolic events are of clinical value, since the Rotterdam Scan Study reported on vascular cognitive impairment related to cerebral embolic events in a community dwelling study22. However, this pathomechanism resulted in a clinical manifestation or progression of cognitive impairment after four years. In short-term and mid-term studies, the contribution of embolic cerebral injury to periprocedural neurocognitive decline is controversially discussed24,25. A recent meta-analysis of 22 studies even questions the “(un)importance of cerebral microemboli”24. With respect to emerging neuroprotective approaches, to the increase of life expectancy, and to the expansion of TAVI to patients at intermediate surgical risk, it is a pivotal finding that embolic events seen with DW-MRI have an impact neither on patients’ self-sufficiency in activities of daily living nor on survival one year after TAVI. However, the impact of cerebral injury on long-term outcome, as well as on the development of vascular dementia, remains to be elucidated.
MEASURES OF CEREBRAL INJURY - CLINICAL
Smith and colleagues reported a stroke rate of 5.1% in patients at high surgical risk at 12 months2. In this cohort, the incidence of a cerebrovascular accident related to TAVI was 6.6%. The risk is slightly higher as compared to the PARTNER cohorts, a fact that could be explained by the limited patient numbers in our study population1,2. In concordance with a recent study conducted by Tay and co-workers, previous cerebrovascular events were associated with an increased risk of TAVI-related embolic events26. This is crucial, since this group further raises awareness on late onset of peri-interventional neurological deficits. Our patients experienced stroke within the “high-risk period”, presenting focal deficits not earlier than six and not later than 48 hours after TAVI. However, none of the patients of this cohort experienced a stroke or a transient ischaemic attack later during follow-up. This can be attributed to the limited patient numbers and the follow-up period. Both patients with TAVI-related major stroke died within the first six months following TAVI. Due to the limited patient numbers, comprehensive conclusions cannot be drawn. However, in our cohort the incidence of minor peri-interventional cerebrovascular accidents was not significantly associated with all-cause mortality after TAVI, whereas major stroke was associated with adverse outcome.
OUTCOME MEASURES
Conventional investigation of health-related quality of life with standardised questionnaires is often regarded as adequate functional investigation after TAVI. However, this method is limited in very elderly patients27. Physicians and interventional cardiologists are often asked to estimate the risk of mental decline, deterioration in daily activities, and loss of self-sufficiency after TAVI. Preservation of the patient’s post-interventional self-sufficiency is a critical endpoint. This is the first study to encompass crucial “everyday” consequences, such as functional status, self-sufficiency at home, and capability to participate in leisure pursuits.
Limitations
This study is mainly limited by the small sample size and the single-site data collection. Interpretation of the data is further impeded due to the DW-MRI drop-outs, which was mainly due to permanent pacemaker implantation post-TAVI. Due to the low event rate, this pilot investigation could not examine the association of TAVI-related subclinical and clinical brain injury with future cerebrovascular events. This study therefore focuses on self-sufficient lifestyle and survival as intriguing outcome measures in an octogenarian population. The limited number of patients further impedes a multivariate statistical analysis identifying independent risk factors for TAVI-related cerebral injury.
Clinical implications
This is the first observational pilot study on the mid-term consequences of cerebral injury on patient outcome after TAVI. Interestingly, the occurrence of silent cerebral embolism seems to have no significant impact on self-sufficiency and mortality within the first year after TAVI. However, long-term follow-up is needed to elucidate fully the impact of silent stroke.
Funding
This trial was supported by the “Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte”.
Conflict of interest statement
E. Grube is a proctor for CoreValve/Medtronic. The other authors have no conflicts of interest to declare.

