
Abstract
Aims: The aim of our study was to compare the impact of implantation of a balloon-expandable transcatheter valve into the inferior vena cava (CAVI) on exercise capacity with optimal medical therapy (OMT) in patients with severe tricuspid regurgitation (TR) and high surgical risk.
Methods and results: Twenty-eight patients were randomised to OMT (n=14) or CAVI (n=14). The primary endpoint was maximal oxygen uptake at the three-month follow-up. Secondary endpoints included six-minute walk test, NYHA class, NT-proBNP levels, right heart function, unscheduled heart failure hospitalisation, and quality of life as assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ). Patients underwent follow-up examinations one, three, six, and twelve months after randomisation. Maximal oxygen uptake did not change significantly in either group after three months and there was no difference between the OMT and CAVI groups (−0.1±1.8 ml∙kg−1∙min−1 vs −1.0±1.6 ml∙kg−1∙min−1, p=0.4995). Compared to baseline, CAVI improved NYHA class, dyspnoea, and quality of life after three months. However, there were no statistically significant differences in the secondary endpoints between the groups. Four periprocedural complications occurred after CAVI, resulting in open heart surgery. Four patients in the OMT group and eight patients (including four after conversion to surgery) in the CAVI group died from right heart failure, sepsis or haemorrhage.
Conclusions: CAVI did not result in a superior functional outcome compared to OMT. Due to an unexpectedly high rate of valve dislocations, the study was stopped for safety reasons.
Introduction
Severe tricuspid regurgitation (TR) is associated with high morbidity and mortality1,2. Medical therapy is often insufficient for adequate symptom relief and patients frequently suffer from effort dyspnoea and refractory peripheral oedema. Despite its high prevalence, isolated surgical repair of TR is rarely performed and perioperative mortality remains high3. Encouraged by the success of transcatheter aortic valve replacement (TAVR) and edge-to-edge repair of the mitral valve, various approaches for interventional treatment of TR have been proposed4. Due to the size and anatomical complexity of the tricuspid valve, there is no currently available percutaneous transcatheter valve system suitable for direct implantation into the native tricuspid annulus. Accordingly, most approaches for interventional treatment of TR aim at bicuspidisation of the valve or annuloplasty5. However, these novel therapies are technically challenging and require excellent intraprocedural visualisation of the valve by transoesophageal echocardiography (TEE)6.
As backflow into the inferior vena cava (IVC) leads to congestive hepatopathy and gastropathy, it is a major component of the pathophysiology of severe TR. We have previously shown that inferior caval valve implantation (CAVI) reduces IVC peak pressure7. We therefore hypothesised that implantation of a transcatheter valve into the IVC may improve symptoms and exercise capacity of TR patients by reducing abdominal regurgitation and congestion. In addition, CAVI is a comparatively simple procedure and can be performed using commercially available products. Furthermore, it can be guided by fluoroscopy and transthoracic echocardiography and therefore does not require general anaesthesia which is usually necessary for TEE guidance8.
Encouraged by a series of compassionate use cases which suggested both technical feasibility and symptom relief7,9,10,11, we designed the randomised TRICAVAL trial to compare optimal medical therapy (OMT) with CAVI. We recently reported the results of the primary endpoint three months after randomisation in a brief research letter12. Here, we provide detailed information on the study population, the primary and secondary endpoints as well as on the extended 12-month follow-up.
Methods
STUDY DESIGN
TRICAVAL was an investigator initiated, prospective, open-label, single-centre, randomised trial comparing OMT with CAVI in patients with severe, symptomatic TR (NCT02387697). Inclusion criteria were NYHA Class ≥II despite established OMT, age ≥50 years, and high surgical risk (logistic EuroSCORE I ≥15% or other contraindications for conventional valve surgery according to the decision of the local Heart Team). OMT was adjudicated by heart failure specialists and defined as medical therapy as recommended by current heart failure guidelines. For patients with preserved ejection fraction, OMT was defined as the maximum tolerable dose of diuretics controlling oedema. The main exclusion criteria included severe left ventricular dysfunction and severe kidney dysfunction (Supplementary Appendix 1). Patients were further screened for anatomic suitability by 3D echocardiography and (following patient number 12) computed tomography (CT) and excluded when the IVC diameter at the landing zone exceeded 31 mm. Patients were randomised using a computer-generated block randomisation. All patients provided written consent. The study complies with the Declaration of Helsinki and was approved by the local ethics committee (LAGeSo, Landesamt für Gesundheit und Soziales Berlin, Germany) and state authorities (BfArM, Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany).
The primary endpoint was exercise capacity, which was determined by quantifying maximal oxygen uptake by treadmill spiroergometry three months after randomisation. Spiroergometry was performed according to the recommendations of the European Association for Cardiovascular Prevention & Rehabilitation13. In order to maintain comparability, all patients were supported to reach the anaerobic threshold defined by respiratory rules or the respiratory exchange ratio (RER)14. Only tests which fulfilled the criteria of target performance were included in our analysis.
Secondary endpoints included NYHA class, six-minute walk test, NT-proBNP levels, right heart function, unscheduled hospitalisation for heart failure, and quality of life as assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) three months after randomisation. Follow-up visits were scheduled one, three, six and twelve months after implantation. Over the course of the study, three patients from the OMT group declined to undergo further follow-up examinations due to deteriorating health. Safety was evaluated according to the Valve Academic Research Consortium-2 (VARC-2) criteria15.
Severity of tricuspid regurgitation was graded as recommended by the European Association of Cardiovascular Imaging and the American Society of Echocardiography16,17, with a special focus on systolic flow reversal in the hepatic veins.
CAVAL VALVE IMPLANTATIONS
Implantations were performed via a right transfemoral venous access under local anaesthesia and guided by transthoracic echocardiography, as described previously9. Unfractionated heparin was given to reach an activating clotting time >250 seconds. Depending on IVC anatomy, a landing zone was first prepared by implantation of a self-expanding nitinol stent (sinus-XL; Optimed, Ettlingen, Germany) into the IVC protruding approximately 5-10 mm into the right atrium. Subsequently, a 23, 26 or 29 mm Edwards SAPIEN XT transcatheter valve (Edwards Lifesciences, Irvine, CA, USA) was implanted into the junction of the IVC and the right atrium (Figure 1). After sheath removal, haemostasis was achieved by Z-suture of the skin and manual compression. All patients were put on oral anticoagulation after implantation. Doppler echocardiography was used to determine the extent of the liver vein reflux by measuring the velocity time integral (VTI) by pulsed wave Doppler in a prominent vein in all patients before and after implantation.
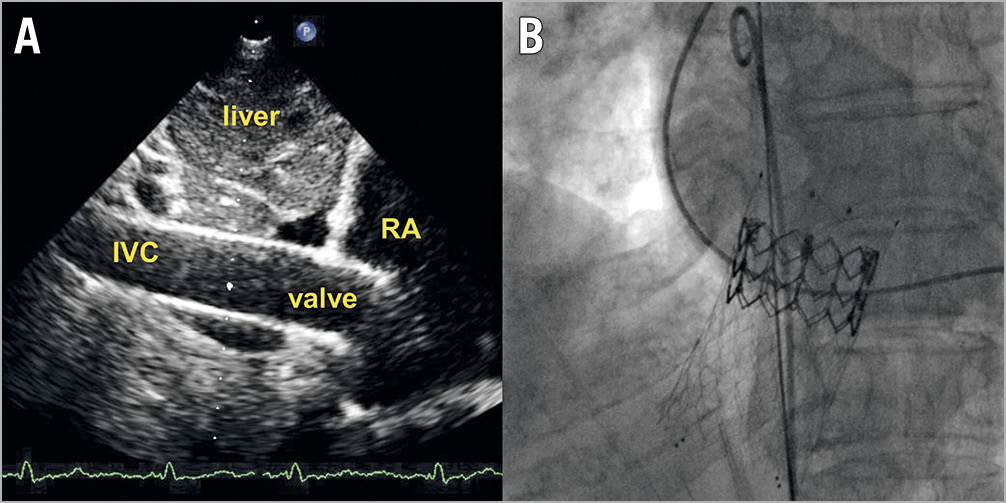
Figure 1. Caval valve after implantation. A) Transthoracic echocardiography (subcostal view). B) Fluoroscopy (posterior-anterior projection). IVC: inferior vena cava; RA: right atrium
STATISTICAL ANALYSIS
Sample size calculation was based on the primary endpoint of maximal oxygen uptake after three months. A difference between the groups of 8 ml∙kg−1∙min−1 was considered clinically significant. To detect this difference with a t-test at a significance level of 5% (two-tailed) with a power of 80% and an assumed standard deviation of 8 ml∙kg−1∙min−1, a total number of 34 patients was calculated to be required (nQuery Advisor 7.0; Statistical Solutions Ltd, Cork, Ireland). To account for a 15% drop-out rate, 40 patients were planned to be randomised.
The primary endpoint was primarily evaluated using a linear regression with group assignment and baseline value as independent variables. As a sensitivity analysis, the unadjusted difference was compared using a t-test. In order to incorporate the follow-up measurements and to investigate time trends, a linear mixed model with maximal oxygen uptake as dependent variable, time, group, and time-by-group interaction as fixed effects and a random subject intercept was calculated. The same approach was used for the MLHFQ but time was modelled with a quadratic effect due to the structure found in the data. Continuous and normally distributed data are presented as means±SD and were compared using the Student’s t-test. Non-normally distributed data are given as median with the interquartile range and were analysed using the Mann-Whitney U test. Categorical data are presented as percentages and were compared using the Boschloo test. Dependent continuous data were compared using paired t-tests or Wilcoxon tests depending on the distribution, while dependent categorical data were compared using the symmetry test. A p-value <0.05 was considered statistically significant although the results of this study have to be interpreted in an exploratory way. All statistical analyses were performed using the SPSS statistical package, Version 23.0 (IBM Corp., Armonk, NY, USA) and R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria)18.
Results
STUDY POPULATION
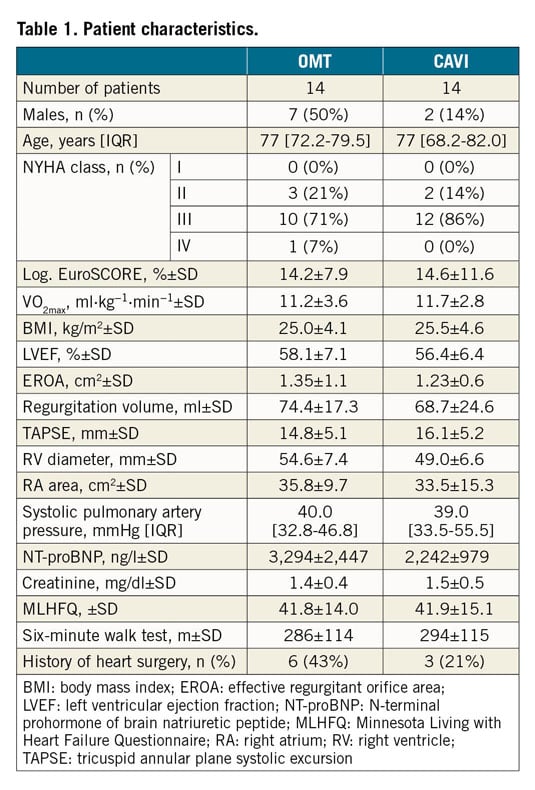
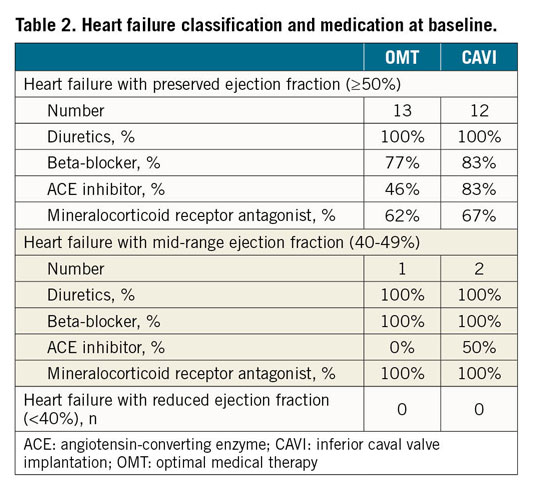
Between January 2015 and November 2017, 28 patients (mean age 75.1±8.5 years) were enrolled and randomised to CAVI (n=14) or continuation of OMT (n=14). Details regarding patient enrolment are given in Figure 2. Patient characteristics at baseline are summarised in Table 1. Details on the heart failure classification and medication at baseline are provided in Table 2. According to a recently proposed grading scheme, four patients (14.3%) had severe, four patients (14.3%) had massive, and 20 patients (71.4%) had torrential TR19.
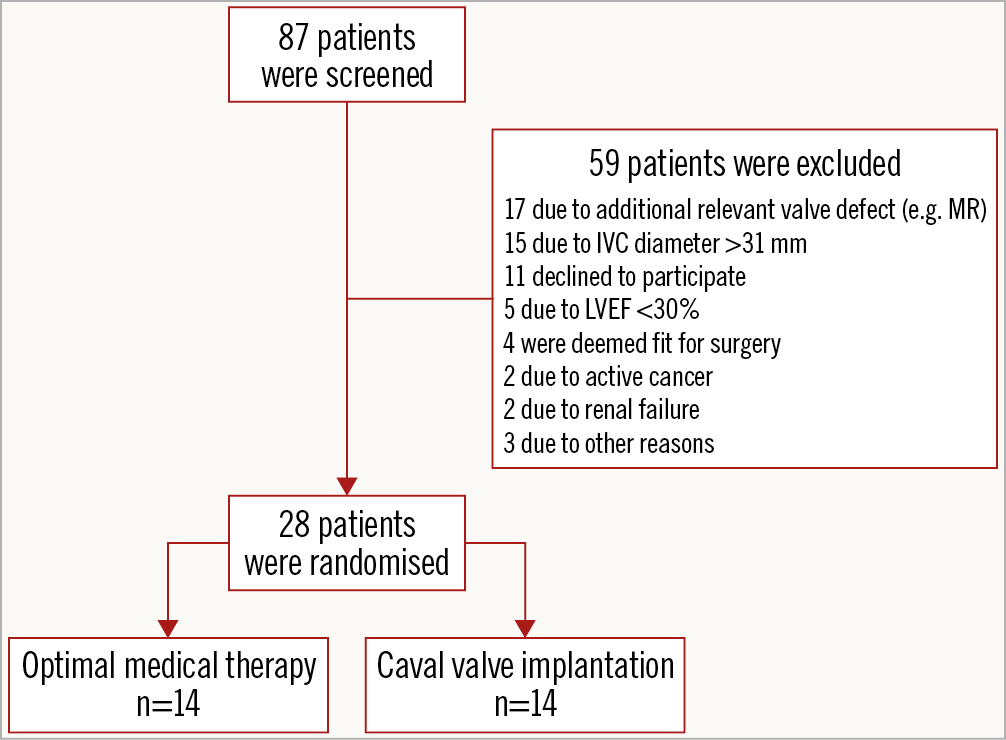
Figure 2. Overview of patient screening and enrolment.
VALVE IMPLANTATIONS
Valve implantations were primarily successful with correct valve deployment in the intended landing zones in all patients. Mild paravalvular leakage was present in two patients (14.3%). Doppler echocardiography confirmed significant reduction of the liver vein reflux in all CAVI patients (VTI 15.4±5.0 cm at baseline vs 5.1±6.6 cm after implantation, p=0.004).
We observed four delayed major complications seven to 48 hours after primarily successful implantations leading to open heart surgery (two cardiac tamponades due to stent migration and two valve dislocations). Patient recruitment was stopped because of safety concerns after the fourth major complication.
PRIMARY AND SECONDARY ENDPOINTS
The primary endpoint, maximal oxygen uptake at month three, did not differ between the OMT and CAVI groups (10.5±3.4 ml∙kg−1∙min−1 vs 11.6±2.6 ml∙kg−1∙min−1, p from baseline adjusted regression=0.4995; unadjusted p-value=0.299) (Table 3). Analysing all follow-up examinations, there was also no significant change over time both in the OMT group (slope 0.09 ml∙kg−1∙min−1 per month; 95% CI: −0.05-0.23 ml∙kg−1∙min−1 per month; p=0.225) and in the CAVI group (slope −0.05 ml∙kg−1∙min−1 per month; 95% CI: −0.19-0.10 ml∙kg−1∙min−1 per month; p=0.530). Furthermore, there was no significant difference in the slopes between the two groups (slope difference CAVI vs OMT −0.13 ml∙kg−1∙min−1 per month; 95% CI: −0.33-0.07 ml∙kg−1∙min−1 per month; p=0.196) (Figure 3).
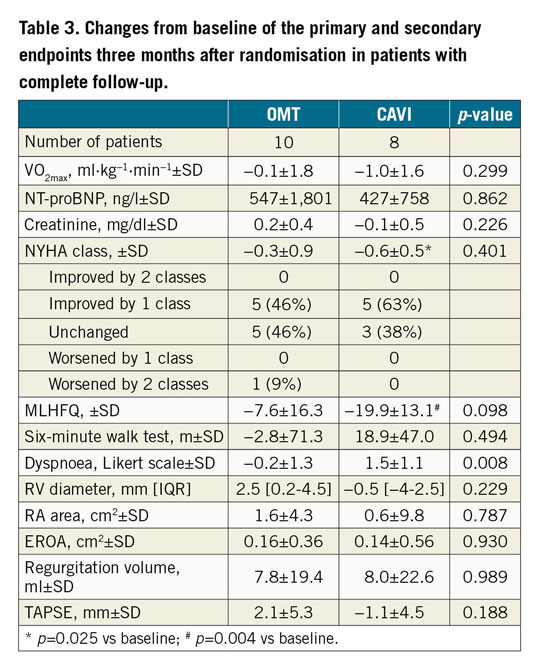
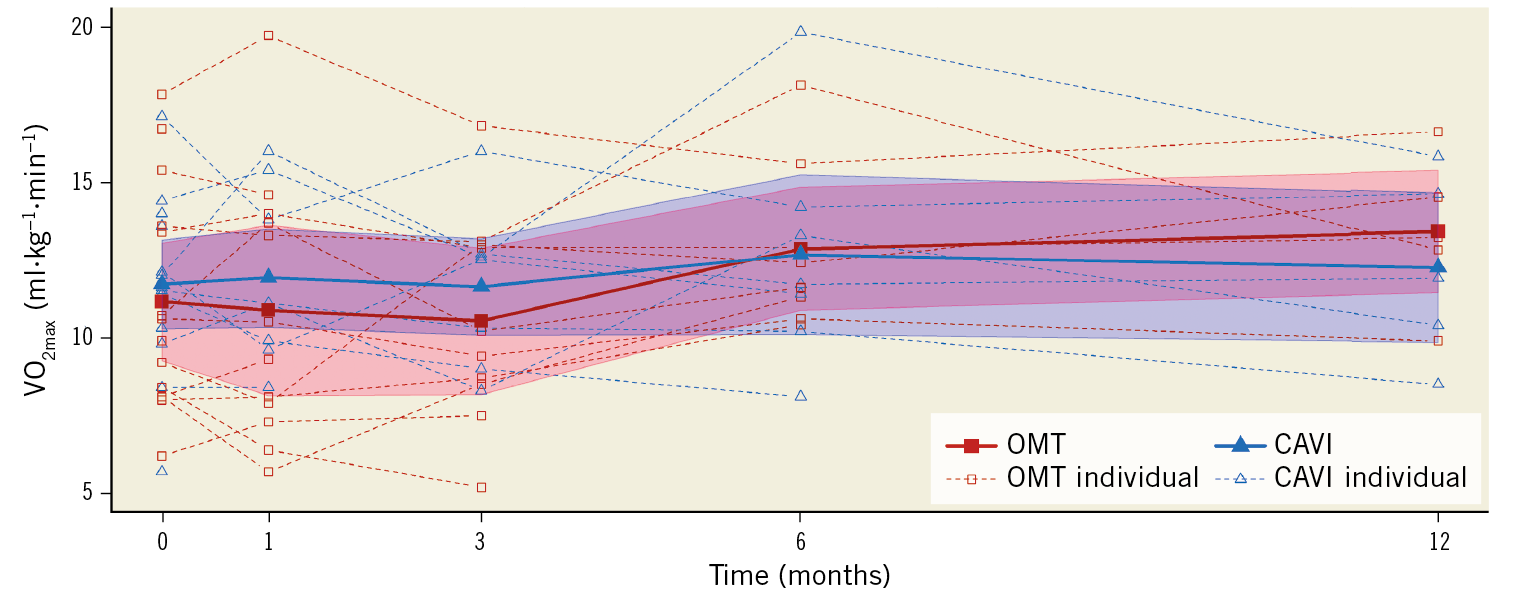
Figure 3. Individual and mean maximal oxygen uptake at baseline and one, three, six, and twelve months after randomisation.
Three months after implantation, CAVI patients with complete follow-up reported a significant improvement of NYHA class (−0.6±0.5, p=0.025). This is in agreement with the subjective assessment of exertional dyspnoea using a Likert scale which showed significant improvement three months after CAVI compared to OMT (1.5±1.1 vs −0.2±1.3, p=0.008) (Table 3). Regarding the change of NYHA class, however, there was no significant difference between the groups over the entire follow-up period (p>0.05 for all follow-up visits) (Figure 4).
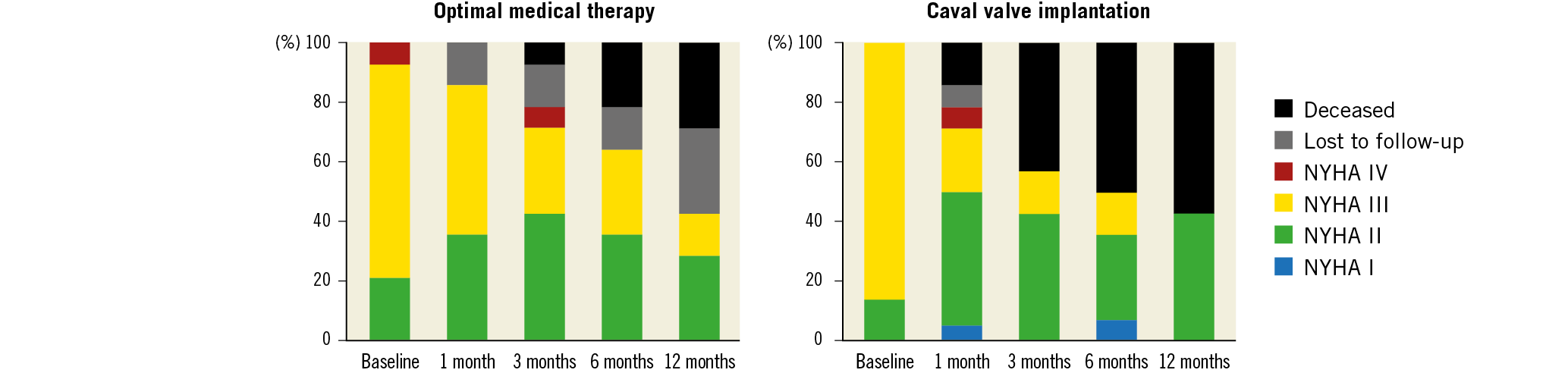
Figure 4. NYHA class at baseline and one, three, six, and twelve months after randomisation.
For the analysis of quality of life measured with the MLHFQ, both groups showed significant improvement over time (p for quadratic time trend=0.040) (Figure 5) with no significant difference between the groups (p for interaction=0.680).
Figure 5. Individual and average quality of life (assessed by the Minnesota Living with Heart Failure Questionnaire [MLHFQ]) at baseline and one, three, six, and twelve months after randomisation. Reduction of MLHFQ values indicates improved quality of life.
In addition, there were no significant differences between the groups regarding the other secondary endpoints with regard to change from baseline (six-minute walk test, NT-proBNP levels, and right heart function) at the three-month follow-up (Table 3).
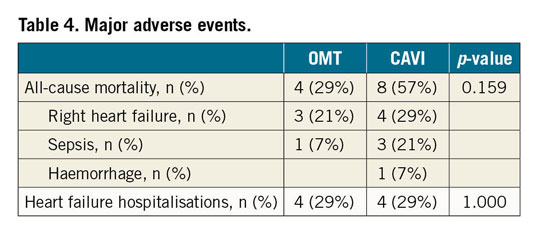
Three patients from the CAVI group (21%) died in-hospital after conversion to surgery from haemorrhagic shock due to resuscitation-related splenic rupture, acute-on-chronic right heart failure, or pneumonia. All-cause mortality after 12 months was 57% in the CAVI group and 29% in the OMT group (p=0.159). There were no significant differences in heart failure hospitalisations between the groups (Table 4). Echocardiography showed normal caval valve function in all CAVI patients one, three, six and twelve months after implantation. There were no major vascular complications.
Discussion
Given the high number of patients with severe TR who are unfit for surgery and remain symptomatic under OMT, the development of interventional treatment options represents a pressing unmet clinical need. Driven by the success of established interventions for left heart valve disease, several approaches have been proposed for the treatment of severe TR5. However, there is a lack of randomised trials as most published data on these new therapeutic options stem from non-randomised case series. Our study represents the first randomised controlled trial analysing the effect of CAVI on exercise capacity in patients with severe TR compared to OMT.
CAVI resulted in a significant improvement of exertional dyspnoea, which was associated with a significant improvement in quality of life. This is in agreement with previously published data from non-randomised studies7,9,10,11 and comparable to the efficacy of other interventional approaches19,20,21,22. Except for the subjective assessment of dyspnoea by the Likert scale, there were, however, no significant differences between the study groups regarding the secondary endpoints, including NYHA class (Table 3, Figure 4, Figure 5). In addition, we observed no significant improvement of exercise capacity after CAVI (Figure 3). However, the interpretation of these results is clearly limited by the low number of patients. Initially, we planned to enrol 40 patients into our study but stopped patient recruitment after the fourth incidence of delayed valve dislocation or stent migration. This was an unexpected finding, as we did not observe similar complications in our compassionate use cases. Valve dislocations are a well-known TAVR complication and have also been described after CAVI7. Nevertheless, the high number of dislocations in our study – despite multimodal assessment of IVC anatomy by CT and 3D echocardiography – raises safety concerns. In stark contrast to the calcified aortic root in TAVR patients, the smooth luminal surface and the fluid load-dependent, variable diameter of the IVC seem to inhibit stable positioning of balloon-expandable transcatheter valves even after pre-treatment of the IVC by implantation of self-expanding stents. In addition, 17% of the screened patients had to be excluded due to an IVC diameter >31 mm (Figure 2). Accordingly, the use of dedicated self-expanding valves or reduction stents suitable for larger IVC diameters should be considered in future studies investigating a possible beneficial effect of CAVI on symptom relief23. This would also allow a bicaval approach with implantation of transcatheter valves in both the inferior and superior vena cava (SVC). In advanced severe TR, the SVC frequently shows a tapered dilatation, which is not suited for implantation of balloon-expandable valves. Therefore – and because backflow into the SVC is often less than into the IVC due to hydrostatic pressure – our study focused on CAVI into the IVC only. In addition, valve implantation into the SVC is frequently limited by the presence of pacemaker and ICD leads.
Echocardiographic follow-up examinations revealed no significant effects of CAVI on the severity of tricuspid regurgitation. Similarly, there were no measurable differences between the groups regarding right heart morphology and function. Despite a significant increase of the difference between the peak v-wave pressure in the IVC and the right atrium before (1.4±3.1 mmHg) and after implantation (11.0±8.2 mmHg, p=0.021), we did not observe an increase of right heart diameters or an impaired right heart function due to a possible pressure overload after CAVI (Table 3).
The question as to which patients are good candidates for interventional treatment of TR remains an unresolved issue. As the majority of our patients had torrential TR with severe dilatation of the right heart, CAVI may have failed to improve cardiac function and morphology due to a lack of potential for reverse remodelling in the advanced stages of right heart failure. Future studies might therefore need to focus on patients who have a high surgical risk but are still in the earlier stages of valvular heart failure.
Limitations
Due to an unexpectedly high rate of complications, the study was stopped for safety reasons, resulting in a low number of enrolled patients. Subjective improvement of symptoms caused by the placebo effect cannot be ruled out as patients were not blinded to the procedure.
Conclusions
Implantation of a balloon-expandable transcatheter valve into the IVC did not result in a superior functional outcome compared to OMT.
Dedicated devices (e.g., TricValve® [P&F Products & Features GmbH, Wessling, Germany], Tricento [New Valve Technology, Hechingen, Germany]) might overcome some of the anatomic challenges observed in our trial23,24. In particular, the risk of valve dislocation needs to be minimised, as the excess mortality in the CAVI arm was driven by patients who underwent cardiac surgery for removal of dislocated valves.
In summary, further studies using dedicated devices in patients in less advanced stages of chronic right heart failure are needed to identify patient subgroups which may benefit from heterotopic tricuspid valve replacement. Currently, CAVI using a balloon-expandable device cannot be recommended due to safety concerns.
|
Impact on daily practice “Heterotopic tricuspid valve replacement” by implantation of a transcatheter valve into the inferior vena cava (CAVI) does not result in a superior functional outcome compared to optimal medical therapy in patients with severe tricuspid regurgitation. Therefore, CAVI using a balloon-expandable transcatheter valve cannot be recommended in patients with advanced heart failure. Further studies with dedicated devices may be needed to identify patient subgroups which may benefit from the procedure. |
Appendix. Study collaborators
Christoph Schöbel, MD; Schlafmedizinisches Zentrum, Universitätsmedizin Essen, Ruhrlandklinik, Essen, Germany. Gert Baumann, MD; Medizinische Klinik für Kardiologie und Angiologie, Campus Charité Mitte, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Funding
The study was financially supported by Edwards Lifesciences (Irvine, CA, USA). M. Laule and F. Knebel received fellowship funding from the Berlin Institute of Health (BIH). B. Hewing participated in the Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health (BIH). I. Mattig was awarded a Kaltenbach scholarship by the Deutsche Herzstiftung.
Conflict of interest statement
M. Laule reports grants from Edwards Lifesciences and Berlin Institute of Health, and speaker and proctor fees from Abbott, Edwards Lifesciences and Medtronic. A. Lauten reports speaker and proctor fees from Abbott, Edwards Lifesciences and Medtronic and is a consultant to P&F TriValve. K. Stangl reports grants from Edwards Lifesciences, and speaker and proctor fees from Abbott, Edwards Lifesciences and Medtronic. M. Thoenes is an employee of Edwards Lifesciences and reports personal fees from Edwards Lifesciences. B. Hewing reports grants from Edwards Lifesciences and from Charité and Berlin Institute of Health. F. Knebel reports grants from Edwards Lifesciences and Berlin Institute of Health. G. Baumann reports grants from Edwards Lifesciences. H. Dreger reports grants from Edwards Lifesciences. I. Mattig reports grants from Edwards Lifesciences and Deutsche Herzstiftung. K. Stangl reports grants from Edwards Lifesciences, and personal fees from Abbott, Edwards Lifesciences and Medtronic. R. Roehle reports grants from Edwards Lifesciences. V. Stangl reports grants from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

