
In this issue of EuroIntervention, Orban et al investigate the impact of a residual >3 mmHg tricuspid valve gradient (TVG) on the outcomes of patients treated with transcatheter edge-to-edge tricuspid valve repair (TTVR)1.
The recently reported results from the TriValve Registry showed that the clinical outcomes of transcatheter tricuspid valve intervention (TTVI) are mainly determined by procedural success and the preoperative stage of the disease (systolic pulmonary pressure and TV coaptation depth)2. The mean post-procedural gradient was not tested as a parameter affecting outcomes. In the present study, Orban et al demonstrate that few patients treated with edge-to-edge TTVR have an average gradient >3 mmHg, and differences in mean gradient after successful TTVI do not affect clinical outcomes at one year in terms of mortality, hospitalisations for heart failure and clinical improvement. The questions that remain open are 1) is the range of normalities applicable in this context, and 2) what does TVG represent in this population of treated patients?
Mean gradients after percutaneous edge-to-edge TV repair: what is the range of normalities?
Given the lack of an evidence-based cut-off until now, an arbitrarily defined TVG of ≤3 mmHg has been considered “acceptable” in TTVR procedures. For native TV pathologies, current international guidelines recommend a cut-off mean gradient of 5 mmHg for expressing a haemodynamically significant gradient3. Also, in the setting of mitral valve repair, a gradient of more than 5 mmHg was related to worse clinical outcomes4. In the context of TV surgical replacement, current guidelines state that normally functioning tricuspid prostheses have a mean gradient of <6 mmHg1,2. For TV surgical repair, a very recently published study shows that, after prophylactic TV repair with flexible ring annuloplasty in the context of MV surgery, TVG remains stable at long-term follow-up, while no relapsing occurs; very low mean TVG was observed also at three- and five-year follow-up (1.65 mmHg at three years and 1.69 at five years)5. In the study of Orban et al, it is clearly demonstrated that the mean gradient after TTVI with the edge-to-edge technique is in the “range of normality”. The median baseline TVG was 1 mmHg and at discharge it increased to 2 mmHg (p<0.001), remaining stable at six- and 12-month follow-up. TVG does not correlate with the primary endpoint when considered as a continuous variable1. Of 145 patients included, only twenty-five patients (17%) showed an elevated TVG >3 mmHg at discharge, and only two patients had TVG more than 5 mmHg. The authors consider that this finding is due mainly to the anatomical and functional peculiarities of the TV which is larger than the mitral valve (MV) and to the right heart which is a low-pressure circulatory system. Moreover, a real comparison between gradients in TTVI and other clinical settings should be applied with caution for two main reasons. First, as is obvious, surgical replacement and repair with a ring – in contrast to edge-to-edge without annuloplasty – have a greater impact on the size of the tricuspid annulus, which is the main determining factor for valve area. Second, patients undergoing TTVI, in contrast to patients undergoing surgery for tricuspid regurgitation (TR), are in a completely different haemodynamic state, and more frequently have end-stage disease1, particularly in the case of secondary TR. For these reasons, a comparison between TTVI and other clinical contexts in terms of TVG has no sense. The set of values of TVG should be established specifically for this group of TTVI patients and their prognostic value should be tested.
Factors affecting mean tricuspid gradient after TTVI
The amount of mean gradient across valves may be altered significantly according to different factors. Figure 1 shows the most important factors that affect TVG. These are always considered in the evaluation of all heart valves and prostheses, but their relative role in the case of evaluation of TTVI with edge-to-edge repair can be greater considering 1) the larger baseline area of the TV as compared with the MV, and 2) the absence of concomitant annuloplasty. As well as in high-flow states (anaemia, hyperthyroidism, sepsis) where TVG can be overestimated, the presence of severe TR can lead to an overestimation of the baseline gradient. In the study of Orban et al, TVG was 1 mmHg, which means that the acute change from 1 mmHg of TVG with severe TR to 2 mmHg with less than moderate TR (successful procedure) is not to be underestimated since the relative increase of gradient, accounting for the slight and rapid correction of overflow due to severe TR, is much greater than 1 mmHg1. Another important factor affecting TV gradient is the preload variance according to respiration. Orban et al assessed TVG according to current recommendations at end-expiration apnoea phase. Moreover, more than 80% of patients included in this study had atrial fibrillation (AF) that can affect heart rate variability and the consequent measurements during follow-up.
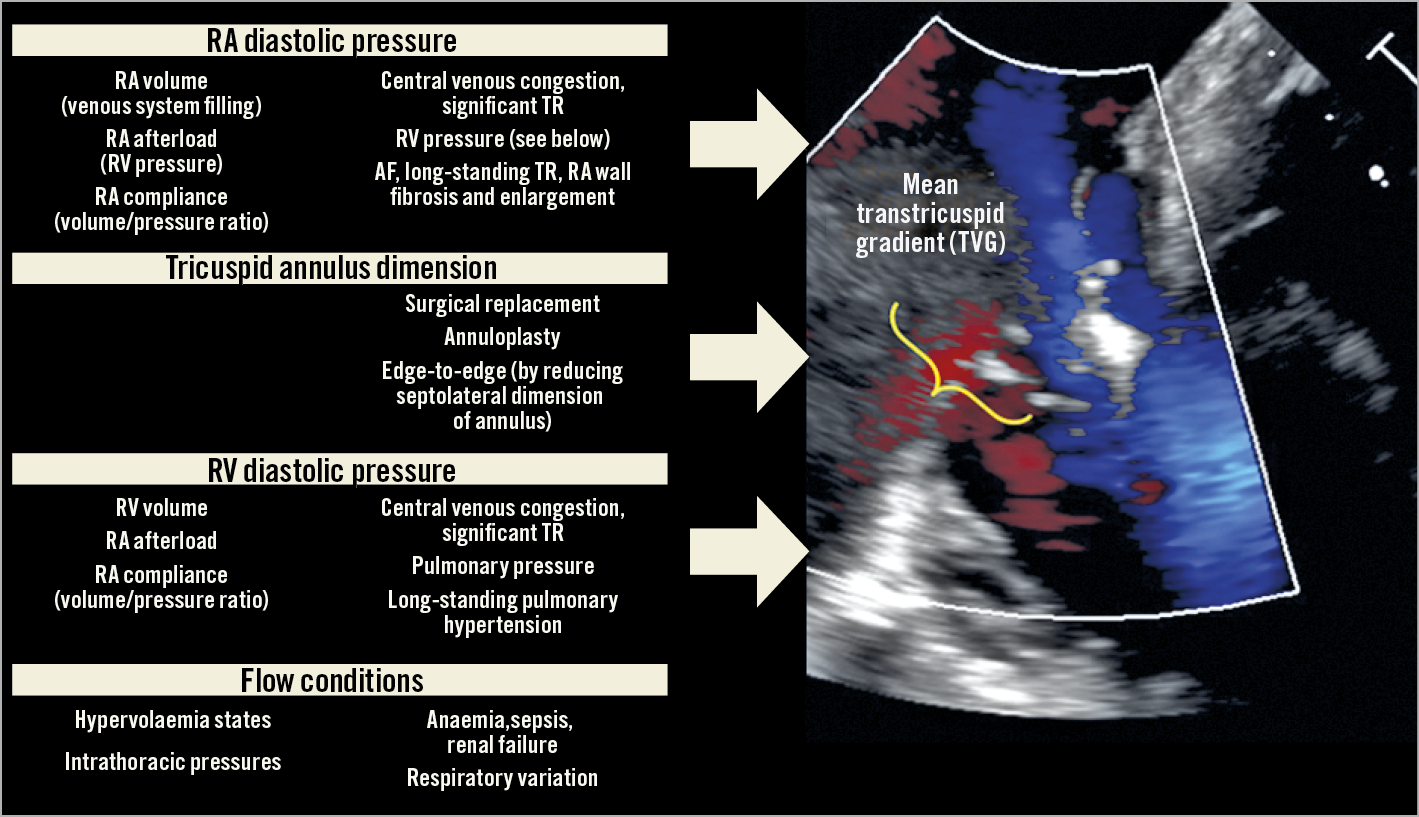
Figure 1. Physiological determinants of transtricuspid gradients, and clinical factors involved.
The “relative compliance of right atrium (RA)-venous system and right ventricle (RV)” is another important factor which can be the result of the aetiology of TR or of the temporal sequence. Different haemodynamic states can occur after successful TR correction. 1) In case of high RA-venous system compliance combined with high RV afterload, the RA-RV gradient is lower; therefore, TVG is lower. This condition is frequently observed in long-standing TR end-stage patients with higher pulmonary vasculature and RV dysfunction. 2) Lower RA-venous system compliance in case of concomitant higher RV compliance can lead to higher RA-RV gradients after TR correction; this condition can be represented by patients with isolated TR, organic TR, post-surgical TR or patients in whom the main mechanism of TR is annular dilatation (long-standing AF or initial, not advanced, RV dysfunction). As proof in the study of Orban et al, patients with higher gradients were younger, more frequently female and with a better glomerular filtration rate, more often underwent cardiac surgery, and also had lower levels of NT-proBNP.
Limited role of evaluation of TVG in clinical practice and intraoperative guidance
Although the evaluation of clinical endpoints in the setting of severe TR and its treatment is still a challenge and one-year follow-up cannot be enough to evaluate the impact of the treatment of TR, the neutral results of Orban et al by correlating TVG with one-year outcomes express the limits of this cut-off for guiding interventions and reaffirm the need for a more integrated evaluation of results. This approach for judging the results should not only be echo-centred but should include the evaluation of the haemodynamic impact of procedural success on single patients. Ideally, the acute reduction of TR would improve the cardiac output and reduce the central venous pressure. These factors can be monitored during intervention, and the finding of an increase in aortic pressure after the reduction of TR is a test of the positive haemodynamic effect of the intervention (Figure 2). Further studies are necessary to demonstrate whether this invasively assessed haemodynamic improvement is related to the outcomes of these patients.
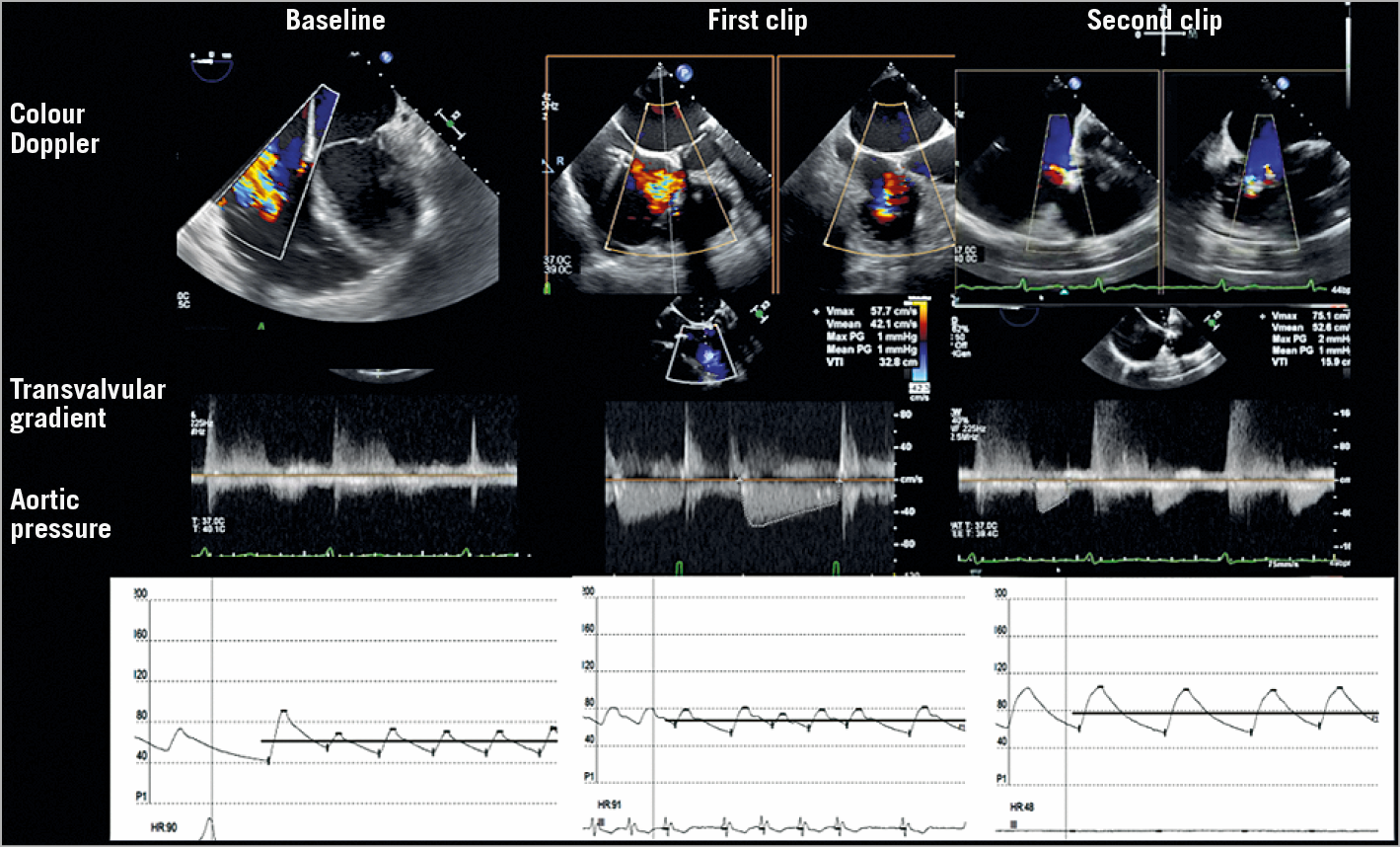
Figure 2. An example of intraprocedural monitoring of percutaneous tricuspid edge-to-edge repair. Top to bottom: colour Doppler assessment of TR, mean gradient (sometimes difficult to measure for extremely low pressure values), aortic pressure (invasive monitoring).
Conflict of interest statement
M. Taramasso is a consultant for Abbott Vascular, 4TECH, CoreMedic and Boston Scientific, and receives speaker fees from Edwards. M. Gavazzoni is a consultant for Biotronik.

