
Over the past decade, thoracic endovascular aortic repair (TEVAR) has increasingly been used to manage Stanford type B aortic dissection due to lower morbidity and mortality as compared with open repair techniques. In the study “In situ diode laser fenestration of aortic arch stent graft during TEVAR of Stanford type A aortic dissection” by Qin et al1, 58 patients with acute or subacute Stanford type A aortic dissection were treated with TEVAR and in situ diode laser fenestration to construct the supra-aortic branches.
Procedural success was achieved in more than 90% of cases. Computed tomography angiography (CTA) demonstrated 100% primary patency of the left subclavian artery (LSCA) and carotid arteries with favourable aortic remodelling during follow-up. Consequently, the authors concluded that in situ diode laser fenestration of TEVAR stent grafts was a safe and effective approach, which could be an alternative strategy to open surgical repair in carefully selected cases with Stanford type A aortic dissection.
Since the first TEVAR device received CE-mark approval in the EU in 1998 and FDA approval in the USA in 2005, the field of aortic endovascular interventions has been evolving rapidly. TEVAR devices are now produced by several manufacturers and are used in most geographical regions for a variety of aortic pathologies, including aortic aneurysms, traumatic transections, and type B aortic dissections. Expert panels in different medical societies have provided guidelines, which should help the practising physician in taking appropriate decisions.
Currently, both the European Society of Cardiology (ESC) and European Society for Vascular Surgery (ESVS) guidelines2,3 on the management of aortic dissection recommend TEVAR as a first-line intervention in case of complicated type B aortic dissection – defined as an aortic dissection starting distally from the LSCA and complicated by rupture, bleeding or compromised blood flow into important aortic side branches (class I; level of evidence [LoE] C). Open surgical repair in such cases should only be considered in case of failure of endovascular management or in cases where endovascular intervention is contraindicated (Figure 1).
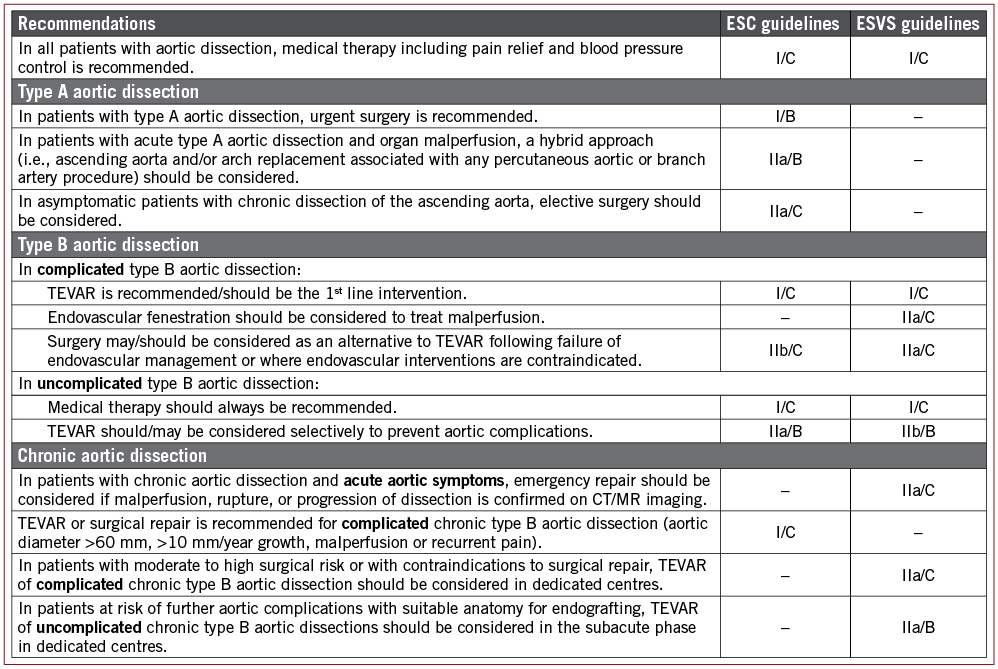
Figure 1. Guideline recommendations from the European Society of Cardiology (ESC) and European Society for Vascular Surgery (ESVS) on the management of aortic dissection, indicating the class of recommendation (I-III) and level of evidence (A-C). TEVAR: thoracic endovascular aortic repair
The role of endovascular treatment is less well established in uncomplicated type B aortic dissection, for which initial management is often conservative with antihypertensive medical management. The ESC and ESVS guidelines state that TEVAR could be considered selectively to prevent late aortic complications (class IIa-IIb, LoE B). However, this recommendation for TEVAR in uncomplicated type B aortic dissections is based on only a few trials with relatively small study populations – the most important studies being the INSTEAD-(XL) (n=140) and ADSORB (n=61) trials4-6. Large randomised trials confirming, and possibly even expanding, the indications for TEVAR in the setting of uncomplicated type B aortic dissection are definitely needed.
Finally, the study by Qin et al in this edition of EuroIntervention1 also suggests a possible role for TEVAR in selected Stanford type A aortic dissection cases. Type A aortic dissection is considered a medical emergency and urgent surgery is recommended following the ESC guidelines2 (class I, LoE B). A hybrid approach, i.e., ascending aorta and/or arch replacement associated with a percutaneous aortic/branch artery procedure, should be considered in cases of type A aortic dissection with organ malperfusion (class IIa, LoE B). In asymptomatic patients with chronic dissection of the ascending aorta, elective surgery should be recommended (class IIa, LoE C).
Clearly, surgical repair is considered the standard treatment option for type A aortic dissection. However, open surgical repair is a complex intervention that often faces serious challenges, which may be problematic for multimorbid older patients. In case of type A aortic dissection not involving the aortic valve and coronary ostia, endovascular techniques may offer a less invasive approach. However, endovascular aortic arch repair remains one of the most difficult challenges in aortic interventions. A proximal landing zone of at least 20 mm is typically required in order to place a TEVAR graft. Even in classic cases of type B aortic dissection, the majority of aortic tears are located in very close proximity to the LSCA, necessitating TEVAR graft extension over the ostium of the LSCA. This may lead to increased risk of complications such as subclavian steal syndrome, spinal cord ischaemia, vertebral artery stroke or arm claudication. Patients with type A aortic dissection have aortic disease, which extends even further into the aortic arch or even ascending aorta, leaving these patients without a suitable proximal landing zone distal to the LSCA. TEVAR in such cases would require not only covering of the LSCA, but also covering of the left common carotid artery (LCCA) or even the innominate artery (IA). Obviously, revascularisation of these major aortic branches would be necessary in such cases, and several techniques have been used to serve this purpose. 1) Hybrid de-branching endovascular aortic repair (elective bypass or transposition of the LSCA/LCCA/IA) is a complex procedure, which often requires prolonged operation and may be associated with substantial risk of various complications (nerve injury, ischaemic and other neurologic complications). 2) Factory branched or fenestrated endografts could theoretically be an attractive option. However, this requires a custom-made device, which is not an option in more acute situations. Endovascular procedures with such devices are also more cumbersome and more prolonged, potentially leading to higher risk of procedure-related complications. 3) Chimney techniques have also been reported as an option for endovascular treatment of aortic arch pathology, an approach which may be easier but which has been associated with substantial risk of endoleak. 4) Finally, in situ fenestration of TEVAR stent grafts can be an alternative for aortic arch reconstruction and revascularisation of the main aortic arch branches. Various endovascular techniques could be considered for this purpose, e.g., penetration by the sharp end of a guidewire, use of a radiofrequency probe or use of an optical fibre and laser-generated energy. Diode laser-assisted TEVAR stent graft modification as described by Qin et al1 seems to be a relatively easy and fast intraoperative approach to revascularise aortic arch branches. In the study, only two fatalities were reported: one patient died of pericardial tamponade during the intervention, and one patient died of severe pneumonia after the intervention. No other fenestration-related complications occurred up to 12 months after the intervention, except for two minor strokes. All in situ laser-fenestrated arteries were patent, as shown by CTA during the follow-up. Only two patients had a type-I endoleak and one other patient had a type-II endoleak. If this technique described by Qin and colleagues proves to be reproducible by other groups, it may be a valuable alternative to open repair in selected patients, and may have potential to be generalised into wider clinical application. However, more studies are needed to confirm the long-term safety and efficacy of this approach.
In conclusion, TEVAR is increasingly being used to treat different types of aortic pathology such as complicated type B aortic dissection, traumatic aortic transection, and aneurysmal disease – especially given the decreased morbidity of this approach compared with open repair. There is also a growing recognition of endovascular therapy as a player in the treatment of selected type A aortic dissections, particularly in patients who are unfit for open surgical repair. Importantly, however, well-designed and well-conducted studies are needed to be able to expand TEVAR indications further in the setting of thoracic aortic disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.

