CASE SUMMARY
BACKGROUND: Four weeks after uneventful transcatheter aortic valve implantation in a fragile woman (80-years-old), the patient represented with an acute aortic dissection type A (AADA, DeBakey type I).
INVESTIGATION: The patient had no signs of malperfusion disorders and no neurological symptoms. CT-imaging revealed a dissection of the ascending aorta with an entry distally above the bare springs of the device. She had a reduced clinical status (frailty, age; log EuroSCORE 22%, EuroSCORE II 6.6%, STS 17.8%.
DIAGNOSIS: AADA, DeBakey type I after TAVI procedure.
MANAGEMENT: A minimally invasive strategy was applied: firstly, open surgery with felt wrapping of the ascending aorta under protection of a cardiopulmonary bypass; secondly, thoracic endovascular aortic repair (TEVAR) with the implantation of a covered stent graft into the ascending aorta via the left groin. Three weeks later the patient was transferred to rehabilitation without complications.
KEYWORDS: emergency, endovascular repair, surgery, thoracic aortic dissection
PRESENTATION OF THE CASE
An 80-year-old, fragile lady underwent a TAVI procedure using a Medtronic CoreValve, due to a severe symptomatic aortic valve stenosis (peak gradient 82 mmHg, area 0.7 cm²). Valvuloplasty with a 20 mm balloon and implantation of a 26 mm device into a 21 mm aortic annulus were uneventful. Intraoperative and postoperative echocardiographic controls showed a sufficient valve prosthesis without relevant paravalvular leakage, gradient or any other relevant pathology. The patient was discharged one week post-TAVI with normal findings in the transoesophageal echo. Four weeks later (i.e., five weeks post-TAVI), the patient suffered from sudden back pain without any further symptoms. Echocardiography revealed an acute aortic dissection type A (AADA) right above the bare springs of the CoreValve device. An additional CT scan showed an AADA extending throughout the aortic arch into the iliac arteries involving the carotid arteries (Figure 1), without any malperfusion disorders. No additional dissection was visible proximal to the entry tear; coronary arteries were unimpaired. So, how should I treat a patient presenting in a reduced clinical status (frailty, 80 years; log EuroSCORE 22%, EuroSCORE II 6.6%, STS 17.8%) and with the complexity of a nitinol-stented valve device in the dissection entry?
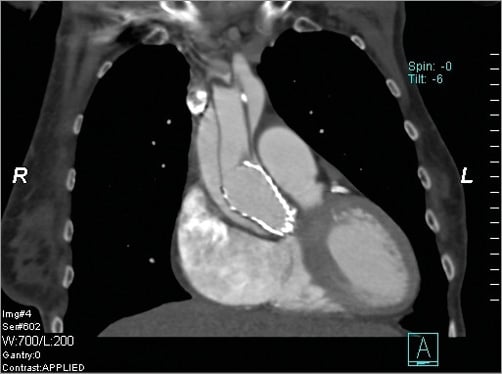
Figure 1. CT scan. An acute aortic dissection type A with an entry right above the metal bare springs of the CoreValve device.
How would I treat?
THE INVITED EXPERT’S OPINION
This is an interesting case example of an acute type A dissection in an 80-year-old fragile high-risk patient, who had undergone successful catheter-based aortic valve implantation using the self-expanding Medtronic CoreValve device. The occurrence of aortic dissection and/or rupture has been described in TAVI literature worldwide as a perioperative complication, and immediate acute conversion plus surgical repair has been reported by multiple groups1-5.
In this specific case, the late complication allows for alternative considerations, since the dissection includes the arch vessels and extends down to the iliac arteries, and the coronary artery flow is protected by the CoreValve.
There are three options to treat the patient and the final recommendation can only be made when one can see the complete CT scan and one can clinically evaluate the patient:
1. If the patient is free of symptoms, medical antihypertensive treatment is an option.
2. If the patient is in clinically better shape since the aortic stenosis has been treated and this has led to a better health status, surgical therapy, i.e., AVR and replacement of the aorta, can be performed.
3. Finally, a transapical stent graft prosthesis could be implanted, anchored through the CoreValve to cover the entry.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Aortic injury is a rare, but severe complication, which may occur acutely in approximately 0.24% of TAVI procedures6, but may also occur later during follow-up7. Management of TAVI-related ascending aortic injury is particularly challenging because of the risk profile and frequent comorbidities of the elderly, frail patient population considered for TAVI. In general, patients with acute dissection involving the ascending aorta (Stanford type A) should be considered for emergency cardiac surgery (ECS). In octogenarians, however, ECS has been associated with significant postoperative mortality rates of 34.9-44.3% and high rates of cerebrovascular complications (stroke: 4.1-12.1%)8-10. As a consequence, fewer elderly patients with type A dissection are managed surgically as compared to younger patients (<70 years)10. Acute aortic injury during TAVI may even portend higher mortality rates after ECS, ranging between 57 and 80%11,12. Outcomes among those with acute type A dissection not receiving surgery (typically because of advanced age and comorbidity, but also patient refusal) were, however, also bleak, with a 30-day mortality of 58% under conservative management13. Therefore, surgery may be considered only for select elderly patients with acute type A dissection10. Less invasive thoracic endovascular aortic repair (TEVAR) by stent graft implantation has been successfully performed in a small number of cases but is difficult due to the anatomical variations of spiral type A aortic dissection14.
In the present case, an 80-year-old fragile woman presented with acute aortic syndrome due to delayed ascending aortic dissection five weeks after an initially uneventful CoreValve TAVI. In view of the published literature and the fragile state of this elderly woman, planning of the further management requires face-to-face evaluation of the patient by the Heart Team and individualised weighing of the treatment options among the Heart Team, the patient and her family. In this critical situation, the patient´s expectations and wishes are an important factor in the decision process. As the patient showed no symptoms of cerebral or peripheral malperfusion, an initial conservative strategy using beta-blockers for blood pressure control and early imaging surveillance may be justified to stabilise the patient. In-depth 3D analysis of the CT scan and additional imaging by transoesophageal echocardiography may be reasonable to evaluate the options of less invasive endovascular repair. As the entry tear appears to be located right above the 40 mm bare stent outflow portion of the CoreValve prosthesis with no evidence of dissection lamella below, there may be an option of sealing the tear by endovascular stent-graft implantation, although TEVAR after TAVI has not been described before. If the patient deteriorates clinically, has refractory/recurrent pain or in case of imaging evidence of disease progression, surgery should be contemplated, on the basis of the Heart Team decision and clear patient motivation.
In summary, it appears reasonable to adhere to a strategy of watchful waiting to begin with. Should the patient develop symptoms or signs of progressive aortic dissection, surgery should be considered but this requires careful face-to-face evaluation by the Heart Team and counselling with the patient and her family.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Transcatheter aortic valve implantation (TAVI) procedures are gaining more importance in the treatment of aortic valve pathologies, especially in the elderly and fragile population. Most complications after TAVI implantation are well known, but an acute aortic dissection type A (AADA) presents a rare challenge. A review of the literature reports aortic dissections in up to 5% after TAVI5,15. Here, we report a patient with an AADA five weeks after TAVI who was treated by a two-stage “minimally invasive” hybrid approach, combining primary open surgery and secondary endovascular intervention.
After diagnosis of AADA, the patient was transferred to the operating theatre. After median sternotomy, cardiopulmonary bypass (CPB) was instituted via the right atrium and left upper pulmonary vein. In order to prevent a sudden rupture of the aortic wall, the ascending aorta was surrounded and downsized by a rigged felt stripe under lowered systemic blood pressure and protection by CPB. The patient was weaned from CPB, and the chest was closed. Hereafter, the patient was immediately transferred to the angiography suite and prepared for a thoracic endovascular aortic repair (TEVAR) procedure. An individually tailored covered stent graft (shortened to 35 mm, Medtronic) was delivered in a retrograde way via the left groin into the dissected ascending aorta (Figure 2). The stent graft was deployed distal to the CoreValve bare springs and proximal to the brachiocephalic trunk (Moving image 1). Subsequent angiography revealed a successful splinting of the dissection in the ascending aorta with a persistent false lumen expanding from the aortic arch into the iliac arteries without any malperfused areas, including the carotid arteries (Figure 3). The patient was brought to the intensive care unit and was weaned from the respirator after two days. Three weeks after this event, the patient had recovered completely without any deficits.
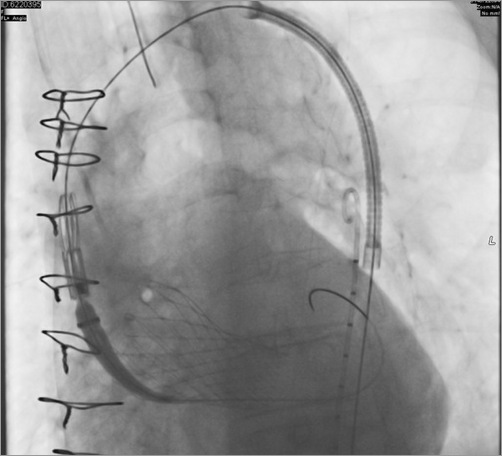
Figure 2. Angiography. Stent graft deployment into the dissected ascending aorta.
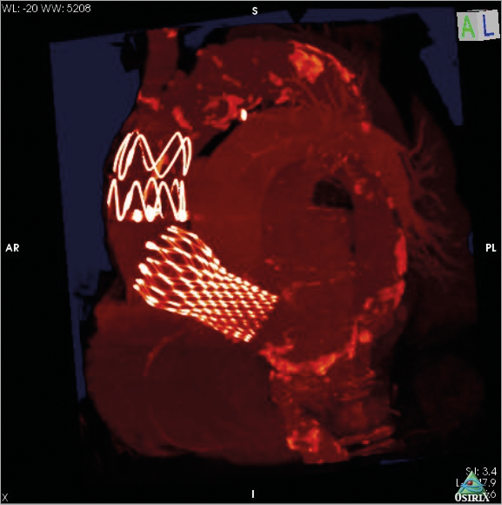
Figure 3. 3D reconstruction after the hybrid procedure. Individually tailored covered stent graft in the ascending aorta between the CoreValve device and the brachiocephalic trunk.
Comment
Until now, very little has been published about AADA after TAVI. Reviewing this case, we have to address the question: “Why didn’t you perform classic open AADA surgery in this TAVI patient?”. After thorough consideration, we had to address the following issues quickly:
1. Which is the best surgical strategy for a frail 80-year-old TAVI patient suffering from an AADA with an elevated surgical risk (log EuroSCORE 22%, EuroSCORE II 6.6%, STS risk of morbidity and mortality 17.8%)?
2. What shall we do with the sufficient CoreValve device in place?
3. How can the ascending aorta be protected from rupture?
4. How should the dissection of the ascending aorta with its potential risk of malperfusions be addressed?
Taking into account that morbidity and mortality in AADA surgery of older patients are significantly increased, it might sound hilarious and insane to perform “classic open surgery” in an AADA patient after a TAVI procedure, especially since patients for a TAVI procedure are either inoperable or at high risk of an open conventional aortic valve replacement - according to the ESC guidelines. However, risk of death in these populations sustaining an AADA is sky-high –about 80%12– and might prohibit any operative approach. Besides the well-known side effects of AADA surgery, an implanted TAVI valve can harm the aorta, the aortic root and the left ventricular outflow tract by extracting the “metal-caged” device, which might not only prolong the operative time but also hamper the implantation of a new aortic valve. However, not treating an AADA is not a satisfactory option for a TAVI patient. Therefore, a minimally invasive strategy as bail-out procedure is urgently required. Since aortic rupture is the most fatal risk, this issue needs to be addressed primarily.
According to the above, conventional AADA surgery should be excluded. Thus, non-biodegradable (polytetrafluoroethylene, PTFE) felt-stripe wrapping of the ascending aorta can reinforce and protect the fragile dissected tissue of the ascending aorta. This less invasive approach in AADA therapy has lately been described by colleagues16. Secondly, malperfusion disorders have to be prevented, which could be achieved by “remodelling” of the dissected ascending aorta by a TEVAR procedure. Stent graft repairs in (hopeless) AADA patients have occasionally been published and were successfully applied in the reported case.
Case conclusion
TAVI-induced AADA is a rare but serious complication in a high-risk patient population, which requires an individually addressed medical strategy. Splinting the dissected ascending aorta from outside and inside in the above-mentioned setting was successfully applied by the combination of primary open cardiac surgery and secondary endovascular stent grafting.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Stent graft deployment in the dissected ascending aorta, right above the CoreValve bare springs.

