Background
Since the first procedure performed in 1986, thoracic endovascular aortic repair (TEVAR) has evolved to become a valuable, minimally invasive treatment option for patients with acute and chronic diseases of the thoracic aorta.
Indications for TEVAR
At present there are no well established guidelines on the particular indications for TEVAR, and it is beyond the scope of this review to provide such recommendations. So far, TEVAR has been successfully used in a variety of thoracic aortic diseases, most importantly in patients with ruptured and non-ruptured descending thoracic aortic aneurysms (TAA, diameter usually >5.5cm in asymptomatic patients), acute complicated type B thoracic aortic dissections (TAD), symptomatic/expanding penetrating aortic ulcers (PAU), and traumatic aortic injury, respectively. TEVAR has also been used in patients with chronic stable aortic dissections, but has shown no benefits over optimal medical therapy in the randomised INSTEAD1 trial.
Difficulties
TEVAR requires advancement of large-bore catheter devices (up to 26Fr) through the femoral/iliac vasculature. Access complications of the iliofemoral axis can usually be avoided by careful pre-interventional planning as well as selection of possible alternative access sites. A sufficient proximal landing zone of “healthy” aorta (proximal neck) of ≥1.5cm is required for successful exclusion of the aortic pathology and sustained long-term success of TEVAR. In short-neck anatomy involving the aortic arch, limited transposition/bypass surgery of supra-aortic branch vessels (hybrid approach) should be performed before TEVAR in order to achieve an adequate proximal sealing zone. Intentional over-stenting of the left subclavian artery (LSA) with the covered part of the stent-graft is usually well tolerated by the patient with respect to arm ischaemia, but significantly increases the risk of neurologic complications such as paraplegia and stroke. Precise implantation of the stent-graft at the intended target location is sometimes challenging due to the windsock effect, which may lead to downstream dislodgement of the partially deployed stent-graft. Newer generation stent-graft devices stabilise the proximal part of the endograft onto the delivery system until final deployment using a “tip-capture” mechanism, which allows for more accurate deployment. Additional strategies involve rapid right ventricular pacing (similar to TAVI).
Methods
Ipsilateral vascular access for advancing the delivery system is obtained either by surgical cut-down to the common femoral artery or percutaneously using a vascular closure device for haemostasis after the procedure. Usually, 5000IU heparin are administered after puncture of the access artery, but in patients with traumatic aortic injury and accompanying injuries (pelvic fracture, organ disruption), TEVAR can even be performed without any heparin in order not to increase the risk of bleeding.
A pigtail catheter is advanced into the ascending aorta using astandard soft 0.035” guidewire. In TAD, it may be challenging to navigate the guidewire into the narrow true lumen of the aorta, which is, however, required for stent-graft placement. TEE or IVUS can be helpful to identify the correct position of the guidewire within the true lumen. It can be helpful to advance then the pigtail without the guidewire to maintain the true lumen position. If the true lumen can not be cannulated from a retrograde approach, snaring a transfemoral guidewire from an antegrade approach via the brachial artery can be helpful.
For intraprocedural angiography, a pigital catheter can be introduced via radial/brachial artery access or from the contralateral femoral artery. From the ipsilateral access, a very stiff 0.035” guidewire (Backup Meier; Boston Scientific, Natick, MA, USA; Lunderquist; Cook, Bloomington, IN, USA) is introduced via a pigtail catheter which is positioned in the ascending aorta. Advancement of the stiff guidewire without a pigtail catheter carries to the risk of aortic injury/perforation. The soft tip of the stiff wire should be positioned in a loop onto the aortic valve. The endoprosthesis is advanced over the stiff guidewire to the thoracic aorta. The first angiography is performed with the delivery system already advanced to the landing zone in order save contrast media and decrease the risk of contrast-induced nephropathy.
When the optimal target position is reached, the stent-graft is gradually deployed. To avoid downstream displacement due to a wind sock effect blood pressure should be lowered pharmacologically (nitroprusside, <80mmHg systolic) or using rapid right ventricular pacing. Rapid pacing is particularly recommended for proximal aortic TEVAR with a landing zone in the ascending aorta or arch. If the intended landing zone is more distal, a less tight lowering of blood pressure should be sufficient. Stent-grafts with proximal tip-capture may also require less strict lowering of blood pressure.
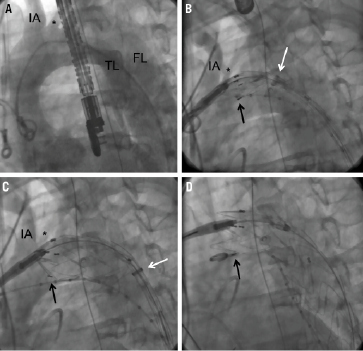
Figure 1. TEVAR in acute aortic dissection complicated by malperfusion syndrome. A. Pre-interventional angiography delineating the dissection anatomy. B. Angiography via the contralateral pigtail catheter during partial deployment of the endograft with the proximal stent springs still attached to the delivery system (“tip-capture” mechanism). Adjustment of the landing position to a more distal position is still possible, whereas proximal advancement is not. In this particular instance, care was taken not to occlude the off-take of the left common carotid artery (*) with the fabric. The black arrow delineates the beginning of the membrane coverage, as indicated by the radio-opaque markers. The white arrow points at the gradually retracted sheath of the delivery system. C. Repeat angiography via the contralateral pigtail during further deployment of the stent-graft. The left common carotid artery (*) is still patent. D. Final release of the proximal stent strut after deployment of the distal graft. The proximal bare stent struts (with membrane coverage) is positioned over the off-take of the left common carotid artery, which does not lead to impairment of cerebral perfusion. The “jailed” pigtail catheter is withdrawn over a standard 0.035” guidewire. IA: innominate artery; *: left common carotid artery; TL: true lumen; FL: false lumen
During deployment of the stent-graft repeated angiographies should be obtained via the contralateral pigtail catheter in order to adjust the position of the endograft to the optimal landing zone. While retraction to a more distal position is still possible with the partially deployed endograft, advancement to a more proximal is not possible. The endograft is finally released when a satisfactory position is achieved. A completion angiography is performed in order to check for proximal type I endoleak (insufficient proximal seal), which usually mandates immediate treatment. Balloon dilatation of the stent-graft may become necessary to improve proximal/distal seal in TAA, but has to be avoided in acute aortic dissection due to the risk of rupturing the fragile dissecting membrane. Even if the stent-graft appears not fully expanded, but compressed by the narrow true lumen, balloon dilatation should not be performed in the TAD as the stent-graft will expand over time due to its self-expanding properties and the induced aortic remodelling processes. In patients with TAD, angiography of the LSA via a radially placed pigtail catheter is very useful for excluding type II endoleaks (retrograde perfusion from the left subclavian artery). Endoleak type IV, due porosity of the fabric, may occur but requires no further action as it will resolve spontaneously.
Conflict of interest statement
H. Eggebrecht has received lecture fees from Medtronic and Bolton Medical.
Reference