
Abstract
Aims: The aim of the study was to evaluate the feasibility, safety, and effectiveness of in situ diode laser fenestration of thoracic endovascular aortic repair (TEVAR) stent grafts to treat Stanford type A aortic dissection.
Methods and results: Fifty-eight patients with acute or subacute Stanford type A aortic dissection treated with in situ diode laser fenestration during TEVAR under cerebral circulation protection with an extracorporeal bypass were reviewed retrospectively. Routine postoperative outcomes were recorded and assessed. Computed tomography angiography (CTA) was performed during the follow-up after 3, 6 and 12 months. Procedural success was achieved in 53 patients (91.4%). The average procedure time was 162±36 minutes. One patient died of pericardial tamponade during intervention, and one died of severe pneumonia after the intervention. Except for two minor strokes, no more fenestration-related complications occurred at 30 days and 12 months after the intervention. CTA imaging demonstrated 100% primary patency for the left subclavian artery and carotid arteries with favourable aortic remodelling after TEVAR during the follow-up. Two patients had a type Ia endoleak and one other a type II endoleak.
Conclusions: In situ diode laser fenestration during TEVAR for type A aortic dissection was found to be feasible, safe, and effective, and may be beneficial as a less invasive approach.
Abbreviations
ACP: antegrade cerebral perfusion
CTA: computed tomography angiography
DSA: digital subtraction angiography
LCA: left common carotid artery
LSA: left subclavian artery
PTFE: polytetrafluoroethylene
TEVAR: thoracic endovascular aortic repair
Introduction
Stanford type A aortic dissection involving the aortic arch remains a catastrophic disease due to high mortality and morbidity rates1,2. Up until now, open aortic arch reconstruction has remained an irreplaceable therapeutic technique3,4, although it shows high morbidity and mortality rates for multiple organ failure5. Compared with open surgery, thoracic endovascular aortic repair (TEVAR) with branched endografts, chimneys, fenestrations, or hybrid-debranching endovascular aortic repair presents an advanced alternative therapy for Stanford type A aortic dissection, extending the option of TEVAR therapy into the realm of the proximal aortic arch in patients with Stanford type A aortic dissection who may benefit from a less invasive approach6-8. Branched-type stent grafts can reconstruct the three branches of the aortic arch9,10, which are customised and limited to use in emergency situations. Additionally, chimney techniques are accepted during TEVAR, which may increase the risk of endoleaks after the intervention11. Furthermore, debranching TEVAR and hybrid-debranching endovascular aortic repair are also available methods; however, they require complex procedures and prolonged intervention times12,13. Compared with the above methods, fenestration, as a rapid and reproducible technique of fenestrating the endograft membrane, presents a potential and effective option to reconstruct the supra-aortic branches14; however, the related reports are limited15-17, because revascularising the three branches of the aortic arch effectively during TEVAR is still a big challenge, especially for the Stanford type A aortic dissection. Differently, in our department, an 810 nm diode laser has been used for the past 15 years to cure superficial venous insufficiency and congenital extratruncular venous malformations18,19. The present study used this diode laser to fenestrate the membrane of aortic arch stent grafts and showed attractive results in repairing Stanford type A aortic dissections.
Methods
PATIENT SELECTION
The study protocol was approved by the Committee for the Protection of Human Subjects at the Shanghai Jiao Tong University, School of Medicine (Shanghai, China). Informed consent was obtained from each patient involved in this study. This was a retrospective observational study. From April 2014 to May 2018, 58 patients with Stanford type A aortic dissection who underwent TEVAR combined with in situ laser fenestration from a zone 0 landing were retrospectively analysed. Patients with aortic artery diseases were included in this study according to critical inclusion criteria, including the damage to the aortic branches and intimal tears adjacent to the aortic/coronary ostia or the proximal seal zone more than 15 mm. The exclusion criteria were as follows: 1) patients with cardiopulmonary and renal insufficiency intolerant to general anaesthesia (the criteria according to the anaesthetist); 2) less than 15 mm distance (seal zone) between intimal tears and coronary ostia; and 3) coronary ostia or cardiac vesicle affected by the dissection. None of the patients had aortic valve failure, pericardial effusion, or other complications.
All procedures were performed under general anaesthesia in a digital subtraction angiography (DSA) operating room. Both carotid arteries, the left brachial artery, and the left or right femoral artery, were exposed surgically under sterile conditions and cannulated with a 6 Fr sheath, 8 Fr sheath, and a 10 Fr sheath (Terumo Corp., Tokyo, Japan), respectively. Intravenous heparin 5,000 U and a 30 U/min drip infusion of heparin were administered. A 100 cm 5 Fr pigtail catheter (Cook Medical, Bloomington, IN, USA) was advanced over a 0.035-inch stiff guidewire (Terumo Corp.) through the femoral artery to the ascending aorta-adjusted coronary ostia, which is an important marker to show the valve location for the next procedure. A 16 Fr sheath (DrySeal Sheath; W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) and a 6 Fr sheath (Terumo Corp.) were inserted into the proximal and distal innominate arteries on different orientations, respectively. Of note, when the 16 Fr sheath core arrived at the tip of the pigtail catheter (15 mm above the coronary ostia), it was pushed forward in tandem with pulling back the sheath core in the ascending aortic arch until next to the coronary valve (15 mm). Then, an 8 Fr sheath (Terumo Corp.) was inserted into the distal part of the 16 Fr sheath, and a 12 Fr sheath (DrySeal Sheath) was inserted into the left common carotid artery (LCA) following the same procedure as the 16 Fr sheath. Cerebral blood perfusion and brain protection were established by an extracorporeal bypass through a vascular shunt connection of the bilateral sheath (Supplementary Figure 1), giving time for the following laser fenestration. Under these systemic circulatory arrests, antegrade and retrograde cerebral perfusions were initiated in the following steps by changing different angles for the blood perfusion.
LASER FENESTRATION PROCEDURE
The diameters of the ascending aorta and arch branches were measured using angiography and computed tomography angiography (CTA) (Figure 1), and the thoracic endografts were delivered via the femoral artery access using the double-curved Lunderquist® wire (Cook Medical), which arrived at the level of the coronary ostia according to the tip of the pigtail catheter (a marker, as previously mentioned). The 21-24 Fr sheath (DrySeal Sheath) was inserted from the femoral artery, and a TAG® or conformable TAG (cTAG) endograft (W.L. Gore & Associates, Inc.) was passed into the descending and ascending aortas. Immediately after the TAG or cTAG endograft had been deployed to cover the dissection tears, a balloon catheter of 4×40 mm (Mustang™; Boston Scientific, Marlborough, MA, USA) was inserted using an 810 nm optical fibre and gently advanced close to the LCA thoracic endograft. Importantly, the fibre tip was beyond the tip of the 1 cm balloon catheter. When the fibre touched the TAG membrane, the resistance of its delivery could be perceived. Then, laser energy was applied with 18-watt energy for 3 seconds to create fenestration without any fibre movement. The laser energy was activated, and an in situ fenestration hole was created. Either the whole laser fibre or the Hi-Torque Supra Core® wire (Abbott Vascular, Santa Clara, CA, USA) was passed through the newly created hole. The balloon catheter was then advanced into the aortic lumen over the fibre. Alternative wires, such as 0.035-inch or 0.018-inch wires (Boston Scientific), were passed through the balloon catheter after withdrawing the laser fibre into the ascending aorta. A 100 cm 5 Fr pigtail catheter (Cook Medical) through the fenestration was used, sometimes followed by rotating multiple angles to judge the true lumen to guarantee the balloon catheter of 4×40 mm in the aortic lumen. After confirming the reverse blood flow, balloon dilatation was performed to enlarge the fenestration using 6 to 8 mm balloons (Sterling™; Boston Scientific). After a 0.035-inch Hi-Torque Supra Core guidewire exchange and endograft predilation using an 8 mm balloon (Mustang), a covered stent (10-13.5 mm in diameter, FLUENCY® Plus; Bard Peripheral Vascular, Inc., Tempe, AZ, USA) was placed through the fenestration, and an angioplasty balloon with a 10 to 14 mm diameter was used to “flare” the branched stent graft portion. After changing the angle of the vascular shunt, DSA angiography was performed to demonstrate the aortic stent and LCA fenestration patency. Innominate artery fenestration was also performed using the above method. After confirming the reverse blood flow, balloon dilatation was performed to enlarge the fenestration and a FLUENCY covered stent of 13.5×40 mm (Bard Peripheral Vascular) was placed and a 12 mm diameter balloon was used to “flare” the branched stent. If the diameter of the innominate artery was larger than 13.5 mm, a modified Talent™ (Medtronic, Minneapolis, MN, USA) leg stent graft was used because no such large stent for the innominate artery was available on the market. After placing the branch stent to prevent bubble stroke, a 50 mL syringe with saline-dilated heparin was used to draw out 50 mL of blood through the carotid artery, which was transfused back. After the aforementioned fenestration, a 6 Fr, 55 cm Cook sheath (Cook Medical) was introduced over a stiff wire to the origin of the left subclavian artery (LSA) by angulation and directional control to fenestrate the LSA in a similar manner (Figure 2). A stable branch reconstruction was confirmed using DSA.
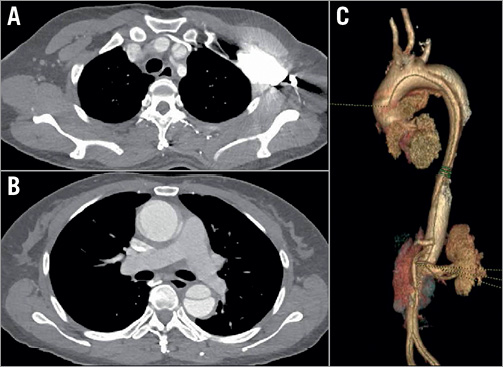
Figure 1. CTA before treatment. A) Cross-section of CTA showing intimal tear location in the aortic arch. B) Cross-section of CTA showing the tear involving the ascending aorta. C) Three-dimensional volume image showing intimal tear at the aortic arch.
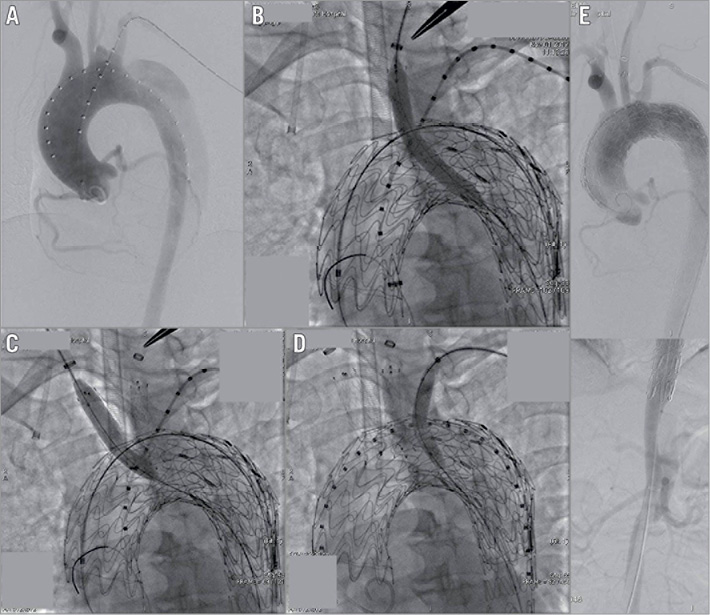
Figure 2. In situ diode laser fenestration of the LCA, innominate artery, and LSA during TEVAR. A) DSA images in the left anterior oblique view. B) In situ diode laser fenestration of the LCA, and one stent implanted in the LCA. C) In situ diode laser fenestration of the innominate artery, and one stent implanted and balloon dilatation performed to enlarge the fenestration. D) In situ diode laser fenestration of the LSA, and one stent implanted and balloon dilatation performed to enlarge the fenestration. E) DSA showing the patency of aortic arch branches.
Finally, the incisions were closed. The patients were then placed under intensive care after the intervention, transferred to a peripheral ward two days after the intervention, and discharged home in good clinical condition.
After the intervention, the technical success, intervention times, clinical outcomes, number of fenestrations of the intimal flap per patient, and complications were recorded. Routine postoperative follow-up CTA imaging was performed to evaluate TEVAR and fenestration patency, endoleaks, and dissection or aneurysm exclusion formation 3, 6 and 12 months after the intervention.
STATISTICAL ANALYSIS
Patient demographics, the morphological presentation of aortic arch arteries, intervention information, clinical outcomes, complications, and follow-up data were analysed using SPSS software, Version 18 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean values ± standard deviation.
Results
PATIENT DEMOGRAPHICS AND PRESENTATION
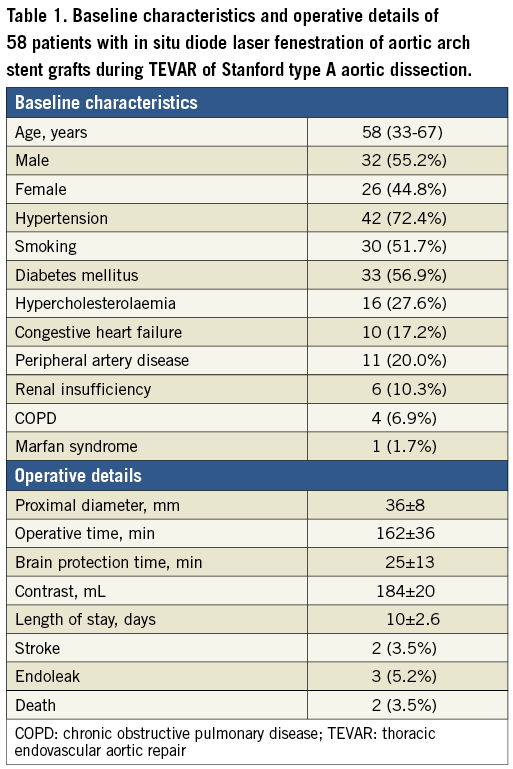
The procedural success of in situ diode laser fenestration during TEVAR of Stanford type A aortic dissection was achieved in 53 of 58 patients (91.4%). Further, 21 patients with acute (≤14 days) Stanford type A aortic dissection were treated, eight of them within 48 hours after the initial admission, depending on the patient symptoms, condition change, and aortic lesion characteristics. Thirty-seven (37) patients were treated in the subacute phase by controlling blood pressure. Two LSA fenestrations were abandoned due to the highly tortuous LSA, with an acute angle between its origin and the aortic arch. Two fenestrations were not achieved due to an acute early take-off of the innominate artery in a type III aortic arch. One patient died of pericardial tamponade during the intervention and one due to severe pneumonia after the intervention, leading to the 3.5% in-hospital mortality rate. No additional deaths occurred during the follow-up (Table 1). All patients complained of severe chest and back pain upon admission. This disappeared two weeks to six months (average, three months) after the intervention during the postoperative follow-up. No patient showed aortic valve failure or other complications.
All the patients underwent TEVAR with in situ diode laser fenestration to revascularise the LCA, innominate artery and LSA under an acute or a subacute emergency situation. Table 1 shows the indications for TEVAR and relative data. Except for stroke occurring in two patients (3.5%) after TEVAR, no myocardial infarction, transient ischaemic attacks, cerebral infarction, respiratory system, renal system, or other neurologic complications occurred during the follow-up period. A follow-up CTA (10.6±5.4 months) indicated that two patients had a type Ia endoleak and one other a type II endoleak; no additional fenestration-related endoleaks occurred, and all patients exhibited a reduction in the aneurysm diameter or the thrombosed false lumen (Figure 3). Furthermore, false lumen thrombosis and subsequent positive remodelling of the aorta were evidenced. All the in situ diode laser-fenestrated arteries were patent according to the postoperative follow-up CTA imaging, which was also evidenced by clinical symptoms.
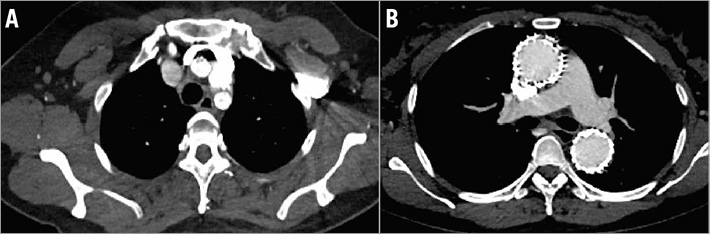
Figure 3. Postoperative CTA at one year presented the patency of three branches of fenestration and stent placement. A) Cross-section of postoperative CTA showing the patency of in situ laser fenestration of the three branches of the aortic arch. B) False lumen thrombosis and subsequent positive remodelling of the aorta were evidenced.
Discussion
The present study shows good technical outcomes with in situ diode fenestration of aortic arch stent grafts during TEVAR of Stanford type A aortic dissection under extracorporeal brain protection, which was designed based on the currently available commercial devices and techniques. CTA demonstrated the complete coverage of primary tears, patent in situ laser-fenestrated arteries, and remodelling of the aorta 3, 6 and 12 months after the intervention in parallel with no stent graft migration and with complete thrombosis formation in the false lumen.
A well-established procedure has been in use in clinics for treating acute Stanford type A aortic dissection20,21. However, conventional surgical procedures are limited for high-risk patients due to high perioperative mortality, such as advanced age, neurological deficits, or other multiple comorbidities22. Although TEVAR in descending aortic pathology, such as type B dissection, has shown promising early and midterm results23, it remains a challenge in type A aortic dissection. Different methods to revascularise the aortic arch branches include elective bypass, transposition of the LSA/LCA, implant of branched or chimney grafts, and fenestrations7,24,25. However, revascularisation of the LSA and LCA requires long operative time and presents high risks for artery ischaemic attacks, nerve injury, and other neurological complications.
In situ fenestrations were employed for the arch arteries and have been reported in both experimental and clinical studies26,27. Different fenestration techniques have been reported: RF probes16, the use of needles or sharp guidewires28, in situ retrograde fenestration, and laser fenestration15.
On the basis of the study by Murphy et al15, laser-assisted in situ fenestration during TEVAR in this study was applied to treat type A aortic dissection. However, a diode laser was chosen because it cured superficial venous insufficiency and congenital extratruncular venous malformations according to our previous reports18,19. The diode laser was more advantageous in terms of biological safety, such as activating a more selective action against blood vessels, compared with previous lasers at wavelengths of 940 or 810 nm, with a selective tertiary haemoglobin peak and a 0.3 mm penetrated depth in the blood. Importantly, the energy generated by this diode laser was absorbed by water and haemoglobin to avoid bubbles, which might cause stroke during fenestration for carotid arteries19. After ex vivo and in vitro experiments, this diode laser was used to fenestrate the stent graft membrane in vivo29. Although a previous study used 10 W energy30, this study used 18 W based on previous experiments29, which might destroy and soften the polytetrafluoroethylene (PTFE) or Dacron fabrics thoroughly. The 810 nm diode laser retained the integrity of the fabric and bare metal portion of PTFE and Dacron grafts and created a round and intact fenestration (Supplementary Figure 2). The soft laser fibre could pass through complex and tortuous aortic arch anatomic variations. In this study, the 810 nm laser provided a rapid and repeatable method for the in situ diode fenestration of endografts during TEVAR, which presented a relatively quick and simple intraoperative endovascular treatment for acute thoracic aortic dissection with stable and safe procedures.
Cerebral protection is a huge challenge during TEVAR fenestration, and has been controversial for many years. Lu et al showed that hypothermic circulatory arrest, unilateral antegrade cerebral perfusion (ACP), and bilateral ACP techniques were effective13. In this study, the cerebral blood perfusion and brain protection were established by an extracorporeal bypass through the vascular shunt connection of a bilateral sheath between the RCA and LCA. Under these systemic circulatory arrests, antegrade or retrograde cerebral perfusion was initiated. One case of stroke after TEVAR in this study might have resulted because of balloon burst due to oversized inflations during percutaneous transluminal angioplasty (PTA), causing the bubbles to enter the intracranial cavity and leading to cerebral infarction (Supplementary Figure 3). A high-pressure balloon is suggested during the fenestration to avoid balloon burst. A 50 mL syringe was used to draw blood when placing the branched stent graft. No myocardial infarction or respiratory system, renal system, or other neurologic complications were found during the follow-up.
Study limitations
The present study had some inevitable limitations. First, this was a retrospective observational study. Second, this study represented only a single-centre experience and not a multicentre experience with the lack of longer-term follow-up data. Finally, the advances in anaesthetic, surgical, cerebral protection, and perioperative care therapies might have affected the clinical results to some extent at different time periods.
Conclusions
In situ diode laser fenestration to revascularise the supra-aortic branches is a feasible and effective option during TEVAR for Stanford type A aortic dissection. In situ diode laser fenestration under cerebral circulation protection with an extracorporeal bypass presents lower fenestration-related neurovascular complications. The high technical success, low mortality, and high patency have extended this application to more patients with Stanford type A aortic dissection using stable and safe procedures.
| Impact on daily practice We evaluated the feasibility, safety and effectiveness of in situ diode laser fenestration of thoracic endovascular aortic repair (TEVAR) stent grafts to treat Stanford type A aortic dissection. Our results demonstrated that in situ diode laser fenestration was a safe and effective approach when patients were selected appropriately and offered an alternative strategy to open surgery. However, further studies with prolonged follow-up, increased surgical numbers, prospective basic research of fluid mechanic changes, and aortic remodelling after TEVAR are required. |
Acknowledgements
The authors thank Meiqiong Qian and Yu Fan, engineers from DSA Therapeutics, for their help with the intervention procedures. We also acknowledge Min Lu and Xinrui Yang for technical support.
Funding
This study was financially supported by the National Natural Science Foundation of China (81601621, 81370423, 81570432, 81600205) and the Natural Science Foundation of Shanghai Science and Technology Committee (Grant No. 134119a2100 and 20124Y132).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Cerebral blood perfusion and brain protection established by extracorporeal bypass.
Supplementary Figure 2. In vitro diode laser fenestration of the PTFE and Dacron stent grafts.
Supplementary Figure 3. A cerebral infarction patient during the in situ diode laser fenestration of the innominate artery after TEVAR.
To read the full content of this article, please download the PDF.

