
Abstract
Aims: The standard approach for thoracic endovascular aortic repair (TEVAR) is transfemoral; however, calcifications and tortuosity of the access vessels might be so extensive as to increase the operative risk markedly or preclude the procedure. This study evaluates the transapical approach as an alternative route for TEVAR in such patients.
Methods and results: From June 2011 to July 2013, the institution’s interdisciplinary board for aortic diseases initially denied TEVAR for eight patients with thoracic aortic pathology due to extensive calcification and tortuosity of the distal vessels. The transapical approach was suggested and approved by the board. All procedures were performed in a hybrid operating room through a left mini-thoracotomy. The stent grafts were implanted in either the proximal descending or the ascending aorta. The deployment was performed under rapid ventricular pacing. Procedural success was 100%. There were no intraoperative complications. One patient needed re-exploration. There was no 30-day mortality. In follow-up, one patient suffered type 1B endoleak, which required surgery after one year.
Conclusions: The transapical approach for TEVAR (TaTEVAR) is a feasible option for patients with distal aorta/iliac vessels unsuitable for transfemoral access. It might be even more beneficial for TEVAR of the ascending aorta.
Introduction
The endovascular treatment for aortic pathology was first described in 1984 by Alexander Balko and colleagues1. This was followed by the pioneering work of Parodi, Palmaz and Barone in 19912, and the extensive work by Craig Miller and the Stanford group3. Since then, endovascular aortic repair has been gaining more attention, becoming a standard therapy for aortic pathology. This modality has been extended to the thoracic aorta, so that thoracic endovascular aortic repair (TEVAR) is now considered a standard procedure for treating pathologies of the descending thoracic aorta.
As experience with TEVAR increased, it became clear that the transfemoral access might be problematic in some cases4. Rupture of access vessels in TEVAR was reported to be as high as 9%5. Subclinical dissection of the iliac vessels was reported to be 38% in some series as well, being related to great tortuosity of these vessels6. This denotes that, despite the femoral access being the standard approach for TEVAR, some patients might present a challenge for stent delivery via this approach, leading to an increased procedural risk.
For this group of patients, a transapical approach might be a solution for safe stent-graft delivery, avoiding dealing with an unfavourable vascular anatomy in such patients. In this study, we report the first case series of patients receiving TEVAR via a transapical approach (TaTEVAR) at our institution.
Patients and methods
From May 2011 to June 2013, 110 patients were presented to our university interdisciplinary board for aortic pathology for evaluation for TEVAR. From the evaluated group, eight patients were found unsuitable for TEVAR due to their aortic or iliac vessel anatomy. The assessment was carried out in a setting involving the university experts in the fields of cardiothoracic surgery, vascular surgery angiology, cardiology, and interventional radiology. Based on our experience with TAVI, the transapical approach for stent-graft delivery was discussed and agreed upon. All patients were included in our university prospective registry. All peculiarities, operative procedures and possible risks were explained to the patients and written consent was obtained. The preoperative, operative, and postoperative data were prospectively collected. A preoperative CT scan was used for procedural planning and stent-graft measurements. Postoperatively, all patients received acetylsalicylic acid therapy (100 mg once daily) indefinitely.
STATISTICS
The results of distribution analysis of continuous variables are presented as mean±SD. The binary variables are presented as percentage of the study population. All statistical analyses were carried out using JMP version 6.0.0 software (SAS Institute Inc., Cary, NC, USA).
PATIENT DEMOGRAPHICS
The mean age was 73±5 years, 60% were male, 25% suffered from diabetes mellitus, and 60% suffered chronic renal failure. The detailed preoperative characteristics are listed in Table 1. All the patients had one thing in common, namely an abdominal arterial system that would not allow an aortic stent delivery system to pass, at least not safely.
PROCEDURE (Moving image 1)
All procedures were performed in our hybrid operating room and imaging was provided by a floor-mounted angiographic C-arm system (Siemens Artis zee; Siemens AG, Munich, Germany). Our standard general anaesthesia was induced by thiopental and maintained by sevofluorane. Analgesia was achieved using fentanyl and muscle relaxation by rocuronium. A radial artery and central venous lines were placed for haemodynamic monitoring. A transoesophageal echocardiography (TEE) probe was also placed. Patients were laid in the supine position and draped in the standard sterile technique. Just before draping, the exact position of the heart apex was determined using transthoracic echocardiography and the site of incision was marked on the skin. A diagnostic angiographic catheter (PIG Super Torque® Plus angiographic catheter; Cordis Corporation, Miami Lakes, FL, USA) was placed percutaneously in the right brachial artery or femoral artery (if possible). A left mini-thoracotomy incision of about 10 cm was made at the site determined earlier by echocardiography, usually in the fifth intercostal space at the mid-clavicular line. After exposure of the left ventricle and the placing of a rib retractor, epicardial ventricular pacemaker wires were placed (TME 68 TVL bifurcated; Osypka, Rheinfelden, Germany). Those would be used later for achieving the rapid ventricular pacing. The exact position of the “tip” of the apex of the left ventricle was then determined by poking it with a finger and observing the indentation of the finger with the TEE. At this site, two pledgeted purse-string sutures were placed. After heparin (5,000 units) administration, the heart apex was then punctured between the pledgeted sutures with an 18 gauge needle, through which a soft wire (EMERALD® Diagnostic Guidewire; Cordis Corporation) was advanced across the aortic valve and into the descending abdominal aorta under fluoroscopic guidance (Figure 1). This was followed by inserting a soft-tipped 14 Fr (30 cm long) sheath (Check-Flo® Performer® Introducer; Cook Medical, Bloomington, IN, USA). With the help of a Judkins right catheter (JR4 Super Torque® Plus angiographic catheter; Cordis Corporation), the soft wire was replaced by a 260 cm long guidewire (Amplatz Super Stiff™; Boston Scientific Corp., Marlborough, MA, USA) which was positioned across the aortic arch and into the descending aorta. Under fluoroscopic guidance, the Judkins catheter and the 14 Fr sheath were removed and exchanged for the endoprosthesis delivery system, which has a 24 Fr outer diameter (in this Moving image 1 case: Relay NBS™; Bolton Medical Inc., Sunrise, FL, USA; all devices that were used in other cases are listed in Table 2). At this point, angiography was performed to determine the sites of origin of the supra-aortic vessels and the planned landing site for the stent. This was marked on the monitor and no further changes were then made to the position of the C-arm. The endograft was advanced to the designated position and again its positioning was confirmed with an aortic angiogram. Rapid ventricular pacing was then initiated until the arterial blood pressure monitor showed a straight line. Next, the endoprosthesis was deployed. Deploying the stent graft under a minimal cardiac output assured a proper placement. Once again, after deployment, the proper position of the graft and the absence of endoleaks were double-checked using digital subtraction angiography together with TEE to exclude any injury to the aortic valve or other cardiac structures. After ensuring a primary operative success, the 24 Fr sheath was withdrawn and the purse-string was tied. The chest was then closed in the anatomical layers and the pleura was drained with a curved drain.
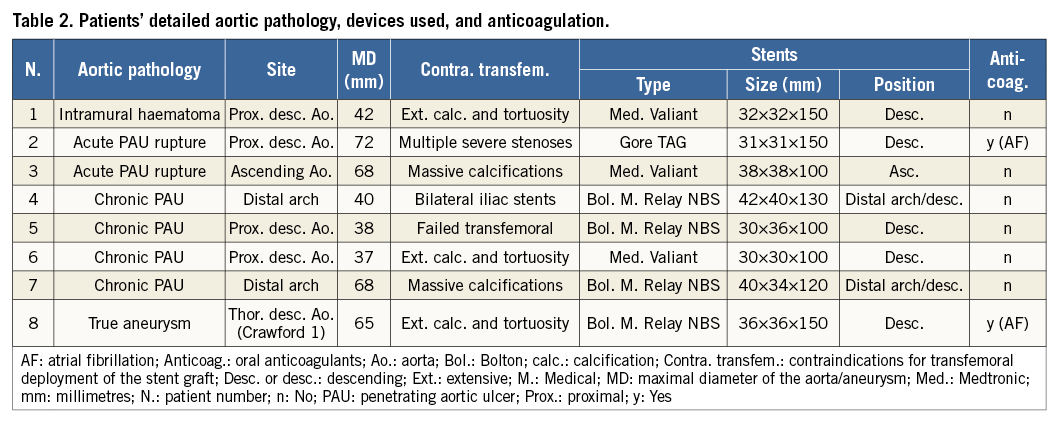
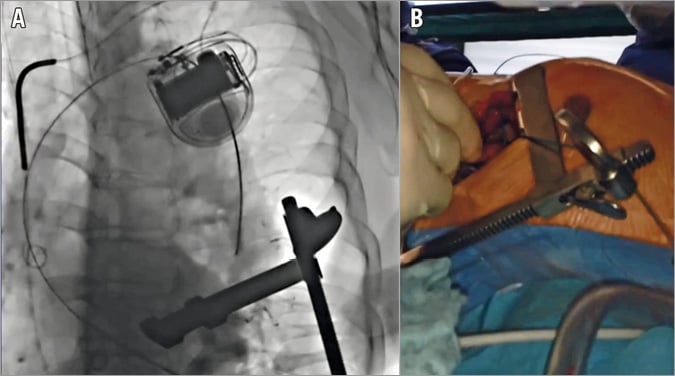
Figure 1. Advancing the guidewire. Fluoroscopy image (A) and intraoperative photograph (B) during the advancement of the guidewire retrograde via the heart apex through the aortic valve into the aorta up to the proximal descending aorta.
Definitions
Procedural success was defined in aneurysms as accurate deployment of the stent graft and the exclusion of the aneurysm itself from the lumen. Procedural success in dissection was defined as the closure of the primary entry tear and/or closure of the penetrating aortic ulcer (PAU) and induction of false lumen thrombosis7.
Intraprocedural complications were defined as any vascular injury or vessel-associated injury (thrombosis, bleeding, retrograde type A dissection, stroke) during TEVAR and within 24 hrs, as well as cardiac complications (perforation of a super stiff guidewire, unintended myocardial injury of any kind)7. Post-procedural complications were defined as any cardiac, vascular or vessel-associated injury occurring after 24 hrs, as well as any neurological deficits or new need for dialysis.
The vascular and cardiac complications were further classified to be access site-related8, or device-related7.
Results
INTRAOPERATIVE RESULTS (Table 3)
All procedures were successful. There were no intraprocedural complications. There were no conversions to open surgery. The mean operative time was 83±19 minutes. Over-stenting of the left subclavian artery was intraoperatively necessary and was performed to establish a safe proximal landing zone in one patient.
POSTOPERATIVE RESULTS (Table 3)
One patient suffered postoperative bleeding and required a second look. The bleeding was found to be access-related (arising from the intercostal muscles). One patient developed an acute renal failure requiring postoperative dialysis. Other than these two patients, the postoperative course was uneventful in all patients. There were no postoperative neurological deficits or 30-day mortality.
FOLLOW-UP
Follow-up CT angiography (CTA) was performed at one week and then six to nine months postoperatively, then once yearly. The mean follow-up period was 36±11 months, ranging from 20-49 months with a total of 24 patient years. All CTAs showed excellent results for all patients over the entire follow-up period, except for the patient who received the TEVAR in the ascending aorta, in whom a type IB endoleak was revealed in the six-month follow-up imaging. The patient underwent an initially successful transapical over-stenting with an E®-xl open stent (Jotec GmbH, Hechingen, Germany). Yet, the following six-month follow-up imaging confirmed a type 1B endoleak. After extensive discussion with colleagues of the aortic board and the patient, a necessary high-risk procedure was agreed upon, in which successful replacement of the ascending aorta could be accomplished, despite marked calcification of the vessel and the disturbed anatomy in the revised procedure.
Discussion
As mentioned before, the transfemoral access is used successfully as a standard approach for TEVAR with excellent results. However, it is not complication-free, and certain prerequisites have to be fulfilled regarding the access vessels. Unfavourable anatomy, such as small lumen, high tortuosity or extensive calcification of the access vessels, is a great challenge for stent delivery via the transfemoral approach, which may lead to higher complication rates. These complications include iliac artery rupture, iliac or common femoral artery dissection with subsequent stenosis or thrombosis, and femoral artery pseudoaneurysm. The incidence of these complications varies in the literature, ranging from 3% to 12.9%4. The rate of access route rupture during TEVAR was reported to be as high as 9% in some series5. It is also reported that patients undergoing endovascular repair and who suffer from a ruptured access point have longer hospital stays and a procedure-related mortality of 11.8%5. Millon and colleagues analysed their data on endovascular aneurysm repair (EVAR) and found a 2.1% incidence of conversion to open surgery, citing iliac artery rupture as the most common cause, with 30-day morbidity and mortality of 36% and 21%, respectively9. Regarding dissection, Tillich and colleagues demonstrated a very high incidence of subclinical iliac artery dissection of 38%6. This was found to be related to excessive iliac arterial tortuosity. In addition, local wound complications at the access site were reported, including haematoma, infection and lymphocele, with an incidence of 1-10%10-12.
The above-mentioned reports do not mean that the transfemoral access cannot be used as a standard approach for TEVAR, as we believe it is still the most appropriate approach, but it denotes that case selection is vital to sort out the group of patients whose access site might be an added risk to the procedure. Many surgical bail-out techniques have been described to enable the advancement of the stent graft, such as “paving and cracking” for small access vessels or the “pull down” technique in cases of severe kinking of the iliac vessels13. Moreover, in cases with severe calcification of femoral or iliac vessels, sometimes it may be necessary to extend the procedure to expose the iliac or even distal aorta to deploy the stent graft14. Recently, several groups reported the use of axillary and carotid arteries while searching for alternative approaches to deploy a stent graft to repair descending aortic aneurysms in patients with complex anatomies15,16.
The transapical approach might just be the answer in such cases. Although it might sometimes be considered a novel concept evolving from TAVI, the transapical approach actually appeared quite early in the history of cardiac surgery. It started as an idea published by Sir Thomas Lauder Brunton in the Lancet in 1902, suggesting the approach for treatment of mitral valve stenosis17. Lauder Brunton never carried out the procedure himself, but his idea paved the way for Elliott Cutler to perform the first transapical mitral commissurotomy which marked the beginning of the history of heart valve surgery18. The transapical approach remained popular until the 1950s with the development of the cardiopulmonary bypass and onset of open heart surgery via median sternotomy. The transapical approach was then resurrected in 2005 in an animal study by Christoph Huber, Lawrence Cohn and Ludwig von Segesser, who suggested it as an access point for transcatheter aortic valve implantation19. This was then implemented for the first time in a human case in 2006 by Samuel Lichtenstein and the Toronto group20. In 2008, Shaun MacDonald and his group in Vancouver conducted an animal study using the transapical approach for TEVAR with encouraging results21. The same group performed the first human case in 200922. This was followed by a few scattered case reports from Philadelphia, Hamburg and Dresden23-25. A small case series has been published reporting three patients with encouraging results26.
Considering the ascending aorta as the new frontier for TEVAR, we believe that the transapical approach might become the standard approach in dissection cases. We realise that there are no evidence-based data at present to support such an approach. Yet, the use of this approach for TEVAR of the ascending aorta presents several advantages. Specifically, the transventricular approach provides not only a non-dissected route, but also a shorter and more direct means for accessing the true lumen of the ascending aorta. This allows easier and more precise placement of the stents compared to other retrograde vascular access routes. Moreover, the transapical approach allows more flexibility regarding delivery system diameters and allows the use of larger delivery systems when needed. Yet, as seen in our case for TEVAR in the ascending aorta, further development is still needed in this direction. This point has been discussed thoroughly in a previous publication from our institute27.
Conclusion
The transapical access for thoracic endovascular aortic repair (TaTEVAR) can provide an additional reproducible approach for stent-graft delivery in patients with unfavourable distal vascular anatomy. The approach might be beneficial in the near future for TEVAR of the ascending aorta.
| Impact on daily practice There are other options available in order to provide a patient with an endovascular solution. Consider the transapical approach a safe alternative for TEVAR in patients with challenging transfemoral access. In endovascular treatment for Stanford type A dissection, it should be considered as the primary approach. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Case illustrating the procedural steps in a transapical approach to TEVAR.
Supplementary data
To read the full content of this article, please download the PDF.
Case illustrating the procedural steps in a transapical approach to TEVAR.

