CASE SUMMARY
BACKGROUND: A 37-year-old man was referred for surgical treatment for a huge thoracic aortic aneurysm (90×80 mm) surrounding an endograft previously implanted in the thoracic aorta. During the procedure, massive haemoptysis occurred with acute haemodynamic instability.
INVESTIGATION: Physical examination, transthoracic echocardiography, thoracic and abdominal CT scan, aortography.
DIAGNOSIS: Haemodynamic instability and massive haemoptysis during a thoracic endovascular aortic repair (TEVAR) procedure for a huge aortic aneurysm.
MANAGEMENT: TEVAR procedure, haemodynamic and respiratory support.
KEYWORDS: aortic aneurysm, massive haemoptysis, TEVAR
PRESENTATION OF THE CASE
A 37-year-old man was referred to our institution for a descending aortic aneurysm associated with a thoracoabdominal aortic dissection. The patient, affected by aortic coarctation, had been previously treated with a surgical replacement of the isthmic aorta with a Dacron graft. After the intervention, he developed a type B dissection of the remaining descending aorta. The aortic lesion was treated with endovascular grafting to cover the entry tear. Three years later, he presented with a huge aneurysm (90×80 mm) which surrounded the endograft (Figure 1). He was referred to our centre and we planned to extend the endovascular grafting of the aorta up to the coeliac artery with a 5 cm overlap of the previously implanted graft (Figure 2).
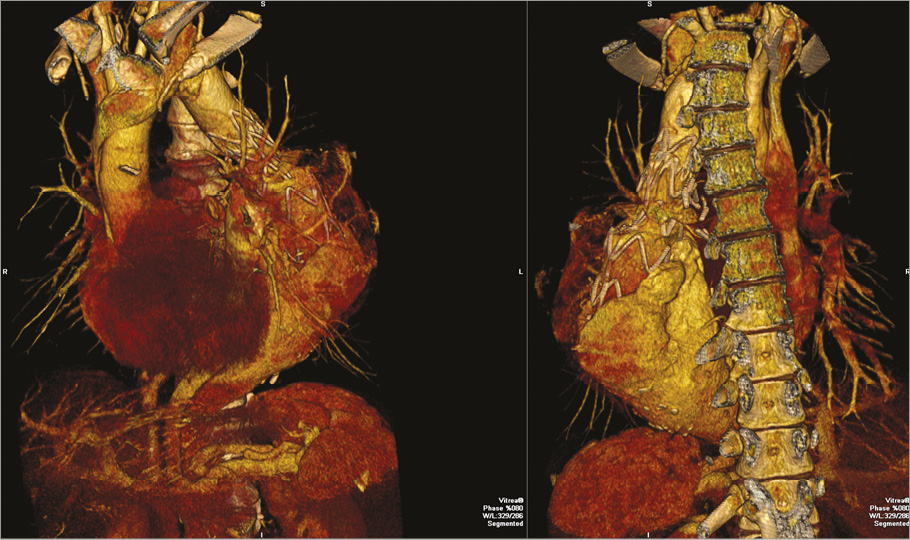
Figure 1. Angio-CT 3D rendering of the huge aortic aneurysm surrounding the previously implanted graft.
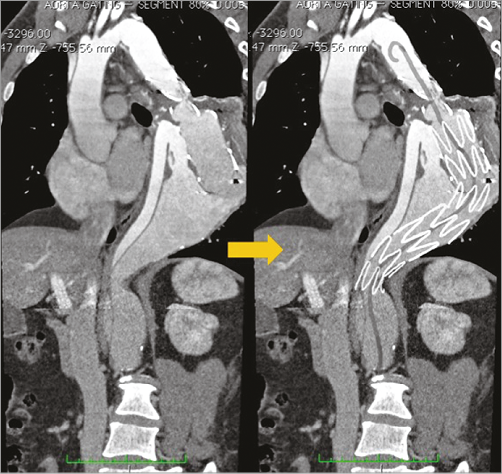
Figure 2. Planned procedure drawn over a CT MPR image.
The procedure was performed in the cardiac catheterisation laboratory using a C-arm angiographic system with digital subtraction angiography (DSA) capability (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
The procedure was carried out under general anaesthesia and with mechanical ventilation. Transoesophageal echocardiography was used to monitor the instrumentation in the aorta and detect potential endoleaks. Right radial access was obtained and a pigtail catheter (6 Fr) (Cordis Corporation, Fremont, CA, USA) was introduced to perform aortography during the procedure.
A right femoral access was obtained, with pre-positioning of two PROSTAR® XL (Perclose; Abbott Vascular, Santa Clara, CA, USA) percutaneous vascular surgical systems.
After many attempts, we advanced a hydrophilic guide into the true lumen through the femoral artery (difficulties were related to the particular conformation of the aneurysm and angulation of the previously implanted graft).
Unfortunately, during these manoeuvres, the patient suddenly became haemodynamically unstable with arterial hypotension (systolic pressure <40 mmHg) and a profuse bleeding was observed from the endotracheal tube.
At this point, what would you do to resolve the critical situation?
How would I treat?
THE INVITED EXPERTS’ OPINION
Aortic rupture during TEVAR for a type B dissection is a rare, but life-threatening complication.
In the case of bleeding with rapid deterioration of haemodynamic status, timely stabilisation and support of haemodynamics is crucial. Given that the intervention was being performed in the cardiac catheterisation laboratory, and a rapid conversion to surgery to obtain aortic cross-clamping was not feasible, the best way to gain bleeding control would be endovascular through an occlusion balloon as already described for ruptured abdominal aortic aneurysm1.
The placement of an occlusion balloon from the femoral access would not be recommended in this case of thoracic aorta rupture because of the several difficulties already experienced in advancing the guidewire into the true lumen. Additionally, the stabilisation of the occlusion balloon above the rupture (ideally inside the previous Dacron graft, at the level of the isthmic aorta) would require the advancement at that level of a long and huge sheath (at least 11 Fr). Such a sheath is needed to avoid balloon dislocation and endovascular cross-clamping failure that may occur when the systolic pressure returns to normal value.
For this particular intra-operative complication we would have considered the placement of an occlusion balloon from a brachial access. Cut-down and vessel isolation can be obtained by an experienced vascular surgeon in a very short time (one to two minutes). This surgical approach allows an 11 Fr sheath to be placed in the brachial artery and a large compliant balloon to be inflated at isthmic level, remaining in a stable position even when the systolic pressure increases. This manoeuvre, together with intensive pharmacological haemodynamic support, would stabilise the patient’s condition. Then, images may be obtained through a pigtail placed below the isthmic aorta, inside the endograft previously placed to cover the entry tear. These angiographic findings may confirm the aortic rupture, probably at the level of the false lumen, which may have occurred during the attempts at true lumen cannulation. The planned procedure of endovascular grafting extension to the coeliac trunk may then be completed.
There is no doubt that the primary endpoint in such a case of aortic rupture is to stop the bleeding and stabilise the haemodynamic status, with the aim of preventing mortality and major cardiac, cerebral, visceral and renal complications. TEVAR by endovascular cross-clamping and endograft extension is evidently the fastest and most recommendable solution in this case, representing the resolution of the acute state.
It would remain to be evaluated whether this endovascular solution may be considered the definitive treatment for this huge aortic aneurysm in a 38-year-old patient, or if a surgical repair with removal of stent grafts and vascular reconstruction of the thoracic aorta should be planned as a more definitive treatment.
As a final consideration we would emphasise how an interventional strategy by covering only the proximal entry tear of a type B dissection (leaving the distal thoracic aorta uncovered) should probably not be considered enough. It is known that one third of the patients treated by this endovascular approach develop further false lumen growth and malperfusion syndrome with a concomitant mortality of 36% at three years2. Nowadays, a more durable solution for a chronic type B dissection seems to be offered by multi-branched endografting3, or by occluding the distal false lumen with an extra-large vascular plug (the “candy-plug” technique4).
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Due to the fact that the aortic aneurysm was close to the left bronchial tree, this life-threatening condition can be reasonably linked to the formation of an aortobronchial fistula (ABF), probably caused by the challenging wiring of the ectatic aorta with a hydrophilic wire.
In this case, after having resolved the patient’s haemodynamic instability, the use of transoesophageal echocardiography and aortography (using a pigtail catheter advanced into the ascending aorta from the radial access) could be used to establish diagnosis5.
ABF treatment usually requires open surgical repair of the thoracic aorta associated with tracheobronchial reconstruction. In this case, thoracic endovascular aortic repair (TEVAR) will exclude the fistulous tract and the pathological aorta with a reduced morbidity and mortality, the risk of which in such an emergency situation is extremely high5.
Endovascular procedures should be performed as soon as possible, under general anaesthesia, in an operating room equipped for prompt open surgical conversion, in case it becomes necessary to de-branch supraortic vessels. The procedure could require extensive stent graft coverage, and a prophylactic placement of the cerebrospinal fluid drainage catheter should be considered.
The operators have already navigated a hydrophilic guidewire in the ascending aorta, at which point a diagnostic catheter can be advanced over it and used to exchange the hydrophilic wire for a 300 cm, 0.035” stiff one.
At this point a large bore (i.e., 12 Fr or more) sheath has to be inserted in one of the femoral accesses potentially to accommodate an aortic balloon, which could be placed at the origin of the fistulous tract to establish haemostasis and give the operators the time to plan a TEVAR.
A pigtail catheter (through the right radial access) can be used to perform an aortogram of the area of interest. Pigtail position is dependent on the predicted landing zone (distal to the subclavian artery in case of landing zones 3 & 4, in the ascending aorta in case of landing zones 1 & 2)6.
A second 0.035” stiff guidewire, introduced in the ascending aorta from the contralateral femoral access through a second large bore femoral sheath, could serve to advance the endograft in the abdominal aorta.
At this point, the aortic balloon can be deflated and retracted into the femoral sheath and, simultaneously, the endograft can be advanced through the contralateral access at the aortic landing zone and be deployed. A completion angiogram and TEE assessment should be performed to confirm the occlusion of the fistulous tract and lack of endoleaks.
Prolonged antibiotic therapy is crucial in this patient, who will need to be followed closely to evaluate the opportunity and timing for a secondary surgical procedure (i.e., bronchial targeted procedure).
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Haemostasis was achieved by inflating a balloon in the descending aorta (Figure 3). Blood transfusions and inotropic support were required to ensure haemodynamic stability. The massive bronchial bleeding was managed by replacing the conventional endotracheal tube with a double lumen endotracheal tube with the aim of protecting the right lung from blood originating from the left bronchial tree, in order to ensure adequate oxygenation, and to provoke a left lung collapse.
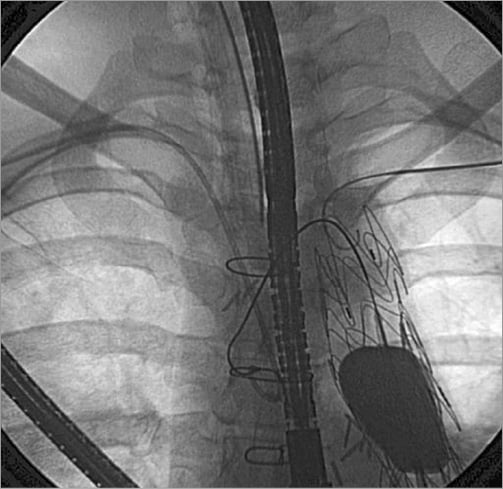
Figure 3. Haemostasis achieved by an inflated balloon in the descending aorta.
To complete the treatment and to resolve this complication, an endovascular stent graft (250×32 mm) (RELAY®; Bolton Medical, Sunrise, FL, USA) was placed between the previously implanted Dacron graft and the aorta just above the coeliac artery, covering the tract of the aorta previously treated as well as the aneurysmatic tract (Figure 4). After positioning of the endograft, bleeding ceased. Aortography showed a residual small type Ib endoleak, with the absence of aortic tears.
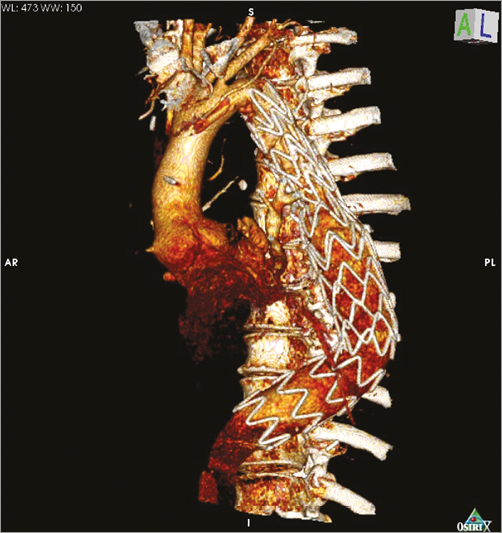
Figure 4. MDCT 3D rendering of the final result.
Transoesophageal echocardiography demonstrated thrombosis of the enlarged false lumen with minimal distal leakage. The patient’s conditions were stabilised and no more bleeding was observed.
A subsequent bronchoscopy revealed a clotted lingular lobe bronchial lesion. The patient was extubated on the fourth postoperative day and the subsequent course was uneventful. At two-year follow-up, the patient was asymptomatic and a CT scan showed complete aneurysm exclusion and lingular lobe bronchiectasis near the aneurysm.
Discussion
Several factors can lead to an aortobronchial fistula (ABF) in patients treated with a stent graft7. Wall stress due to stent graft and inflammatory adherences around the aorta can create strict adhesions between the aorta and the bronchial tree, and the wall of bronchus can become thinner because of the mechanical strain due to continuity with the metallic struts of the stent graft8. In this case, an acute intraprocedural bronchial bleeding occurred. Two conditions had a central role: the aorta was tortuous and presented adherences with the bronchial tree, and the stent graft previously implanted was very close to the bronchial tree. Therefore, we hypothesise that the complication was caused by the intraprocedural manoeuvres. These probably led to an excessive stretching on the previously implanted prosthesis adherent to the aorta and bronchus, resulting in an excessive mechanical stress on the arterial wall and in a subsequent ABF formation.
The pulmonary bleeding was successfully managed by collapsing the involved lung9,10, ensuring adequate oxygenation through the other lung, and by an endovascular clamping of the aorta with an endovascular balloon. These manoeuvres allowed us to block the bleeding and stabilise the haemodynamic conditions of the patient during the preparation for the endograft. The prompt coverage of the ABF with a new stent graft longer than that originally planned guaranteed an effective definitive treatment. The quick inflation of the endovascular balloon and the subsequent placement of the endograft led to the successful resolution of this dramatic case.
Conclusion
ABFs are rare long-term complications of TEVAR linked to aortic anatomical configuration, aortic wall mechanical stress, and migration or structural damage of the graft. In this case, intraoperative ABF development was caused by a strong manipulation of the aorta and the presence of a previously implanted stent graft. Prompt management with aortic endoclamping and lung collapse associated with a long stent graft placement allowed us to stabilise the patient, to arrest pulmonary bleeding and to exclude the ABF completely. A multidisciplinary team approach is mandatory to achieve success in complex and complicated cases.
Conflict of interest statement
The authors have no conflicts of interest to declare.

