
Abstract
The efficacy of an IVUS-guided stent implantation strategy to improve acute results and clinical outcome has been described previously. OCT is another technique which allows high-resolution intracoronary imaging. However, the use of invasive imaging modalities to guide PCI has, as yet, played a limited role in current clinical practice. This may be partly explained by the expertise required for interpretation and clinical decision making. We present a novel technology which enables real-time co-registration of OCT images with angiography. This will simplify matching cross-sectional images to their geographic position on the angiogram, thereby facilitating imaging-guided PCI.
Introduction
Coronary angiography is the only intuitive, easy-to-use, universal imaging modality available to the interventionalist for decision making during device implantation. To provide complementary value to angiography for procedural guidance, novel imaging modalities ideally have to be integrated on the angiographic roadmap. A large body of clinical studies has demonstrated the efficacy of an intravascular ultrasound (IVUS)-guided stent implantation strategy to improve acute results and clinical outcome1. Optical coherence tomography (OCT) is another technique which allows high-resolution intracoronary imaging. Both modalities can be used in selected patients to optimise stent implantation as stated by the current guidelines2. Yet, the use of invasive imaging modalities to guide PCI has played a limited role in current clinical practice due to the extra time, difficulties in matching the cross-sectional images to the angiographic location and the required operator expertise. We present a new OCT technology (OPTIS™ Integrated System; St. Jude Medical, St. Paul, MN, USA), which enables real-time co-registration of OCT images with angiography. Such an online integrated system might improve clinical workflow and decision making. This could be of special value with bioresorbable scaffolds, which have poor angiographic contour delineation and require accurate size matching.
Relevance of co-registration
Coronary angiography does not always allow precise determination of vessel anatomy, with previous studies demonstrating that it tends to underestimate both lumen dimensions3 and the boundaries of lipid core plaque4. This mismatch in sizing and lesion coverage has been associated with stent failure and impaired clinical outcome. OCT guidance could overcome these limitations in PCI; however, it is challenging in daily practice. It remains difficult quickly and reliably to match the geographical position of the cross-sectional OCT images on angiographic views, especially in the absence of anatomical landmarks, or in angiographic foreshortening, angulation, and vessel overlap, thus limiting the use of OCT mainly to large experienced centres. Translating a desired implantation strategy obtained by accurate OCT measurements directly into the angiographic monitor might result in better acute and long-term clinical outcome. Co-registration of invasive imaging and angiography has recently been proven feasible5 and is currently the subject of intense development efforts. As a result, co-registration systems are available for both OCT (Lunawave®; Terumo Corp., Tokyo, Japan, and OPTISi; St. Jude Medical) and IVUS (IVUSmap; Siemens AG, Munich, Germany, and SyncVision®; Volcano Corporation, San Diego, CA, USA) as well as software solutions designed to co-register invasive images to the angiographic roadmap (Medis, Leiden, The Netherlands, and Pie Medical, Maastricht, The Netherlands).
OPTIS Integrated System
The basic principle of the OPTIS integrated (OPTISi) angiographic co-registration system consists of tracking the radiopaque lens marker of the OCT catheter on a cine acquired during OCT pullback (Figure 1). The software of the OPTISi system directly displays a matched side-by-side view of OCT and angiography in the cathlab and eliminates the need for currently used stand-alone, mobile OCT carts (Figure 2). A small white marker is directly projected onto the angiogram, to pinpoint the corresponding site of the displayed OCT frame. The co-registration allows “mapping” of coronary lesions on the angiographic roadmap (error margin ≈1 mm). However, precision and incidence of co-registration failure in a clinical setting have not yet been established. Both might be potentially negatively affected by cardiac and respiratory motion artefacts, vessel overlap or incomplete distal contrast filling in tight lesions when the OCT catheter limits flow. Currently, the level of co-registration confidence is indicated by a colour-coded bar (red=low; orange=high, within the pre-specified margin of 1 mm). Instant automated measurements of reference lumen dimensions and lesion length can be effective for accurate stent/scaffold selection. Direct tableside controls allow the physician to drive system operations autonomously.
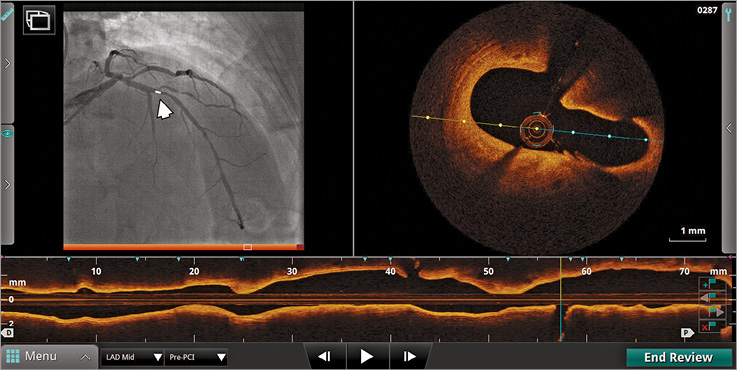
Figure 1. On-line display of co-registration on a catheterisation laboratory screen. The small white marker projected on angiography (left, arrow) corresponds to the current frame indicator in cross-sectional and longitudinal OCT views.
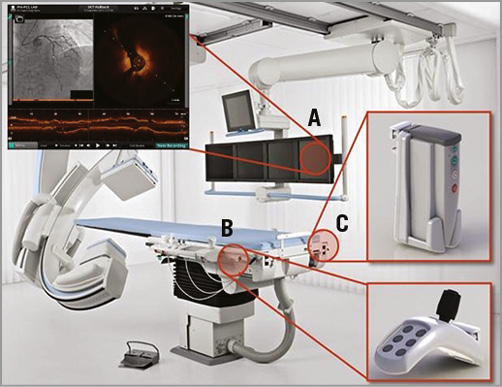
Figure 2. Architecture of the angiography-OCT co-registration system. A) Set-up of the integrated system in the catheterisation laboratory with side-by-side view of OCT and angiography on the monitors. B) A tableside controller within reach of the operator. C) A DOC holster at the table.
OCT data display
The system offers several different options to display the acquired OCT data, in addition to cross-sectional and longitudinal mode (L-mode) views. The automated lumen profile feature visualises in a graphical way the true lumen dimensions (based on a frame-by-frame border detection algorithm) across the entire length of the pullback (with a maximum length of 75 mm). This approach provides user-independent, accurate information on lumen size, vessel tapering and disease extension in a similar way to an angiogram, but with much higher precision. Key stent planning metrics are immediately displayed, including minimal lumen area and percent area/diameter stenosis. Based on the lumen profile information, it is easy for the operator to select proximal and distal normal reference frames, with simultaneous display of the distance between references (lesion length). Most importantly, this approach does not suffer from the typical limitations of angiography such as geometric distortion due to eccentricity, angulation, foreshortening or vessel overlap. An immediately available 3D reconstruction, synchronised with cross-sectional, L-mode and lumen profile views, can be displayed in parallel for more comprehensive visualisation of complex anatomy (Moving image 1).
The latest software release also supports visualisation of reference areas on the angiographic roadmap, as selected by the operator in the lumen profile view, further facilitating intravascular imaging-guided stenting. Furthermore, the software is now also able automatically to detect metal stent struts and highlight struts that are malapposed with a pre-specified distance. This allows immediate visual assessment of the result of stent implantation (Figure 3).
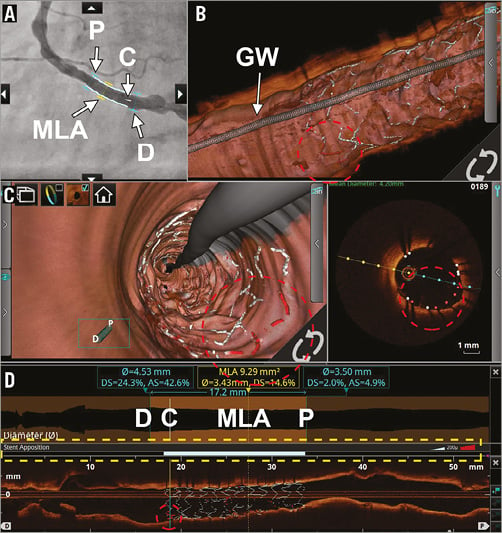
Figure 3. Visualisation options for stent assessment. Co-registered visualisation options of a stent that was implanted in the right coronary artery. A) The angiographic roadmap with markers of the proximal (P) and distal (D) reference segments, minimum lumen area (MLA) and current frame (C). B) A 3D representation of the stented coronary artery, clearly showing the guidewire (GW) and the fish bone structure of the stent, with correctly apposed struts in white and malapposed struts in red (red dotted circle). This is also represented in the flythrough mode and the conventional cross-sectional OCT image (C). In panel D, the lumen profile and longitudinal view, respectively, can be appreciated. The latter also shows the stent contour. Please note the stent apposition bar; even though malapposed struts can be seen, none of them exceeds the pre-specified distance (200 microns) as is represented by a fully white apposition bar (yellow dotted rectangle).
Tips to ensure good angiographic co-registration
1. Select the most appropriate angiographic view, ensuring that vessel foreshortening and overlap are minimised. When selected, maintain the same view at pre and post procedure.
2. Acquire a good quality cine during OCT pullback, ensuring that the cine field encloses the entire target vessel.
3. Ensure that cine captures the entire time duration of the OCT pullback. Start recording just prior to contrast injection and continue until just after completion of the OCT pullback. Inadequate cine lengths will impede co-registered display of angiographic and OCT images and might require repeating the pullback.
Preliminary experience
To demonstrate the potential clinical benefit of angiography-OCT co-registration, we present two cases where co-registration was used to guide PCI and optimise stent implantation. Figure 4 describes the use of pre-procedure OCT for assessment of lumen dimensions and of plaque distribution and composition in a non-ST-elevation myocardial infarction (NSTEMI). Landing zone selection was performed on OCT by identifying the most normal-looking segments proximal and distal to the lesions. After assessing lumen diameter and lesion length by OCT, an Absorb 3.0×28 mm (Abbott Vascular, Santa Clara, CA, USA) bioresorbable scaffold was implanted, leading to a satisfactory post-implantation result. Figure 5 illustrates the use of OCT-angiography co-registration for identifying adverse intracoronary features undetected by angiography and guiding stent optimisation.
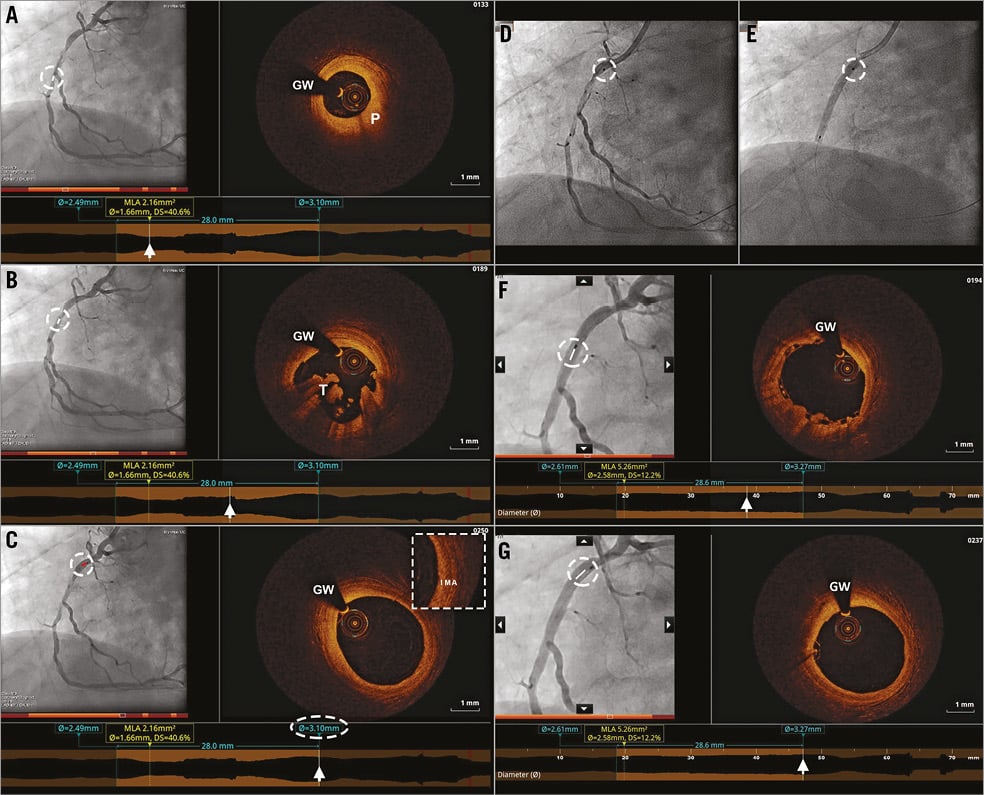
Figure 4. Angiography-OCT co-registration for decision making pre and post PCI in NSTEMI. A) - C) Co-registered angiographic and OCT images in a right coronary artery after predilatation, showing a fibroatheroma (P) with thick fibrous cap, thrombus (T) and an appropriate proximal landing zone with minimal disease. D) & E) This proximal landing zone was used to guide bioresorbable scaffold implantation on angiogram. F) & G) Post-implantation co-registered angiographic and OCT images showing a good implantation result and the absence of edge dissection (G).
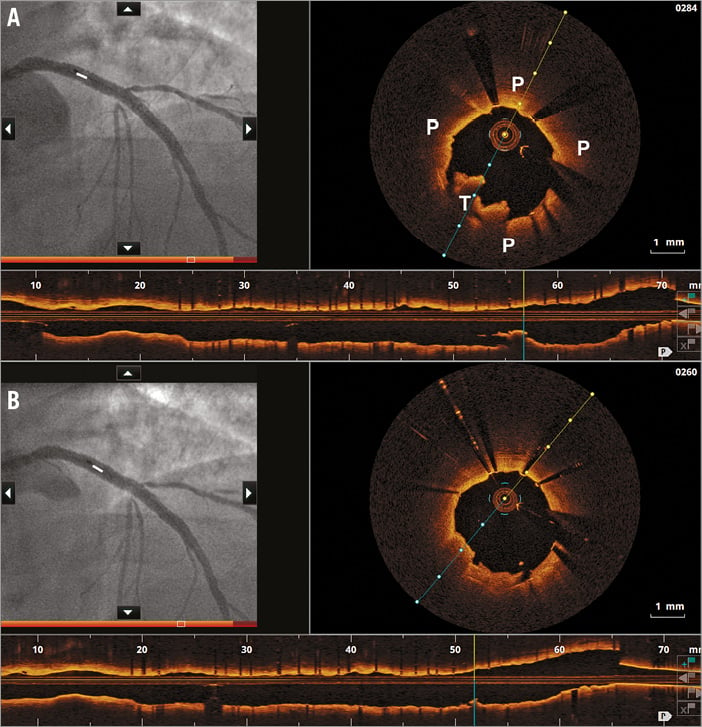
Figure 5. Optimisation of stent implantation in NSTEMI. OCT-angiography co-registration of left anterior descending artery immediately post stent implantation (A). The white marker on the angiographic profile corresponds to the site of plaque (P) and thrombus (T) protrusion by OCT. By using the marker position on the angiographic screen, OCT-guided post-dilation with a larger 4.0×8 mm non-compliant balloon, to correct for mild stent underexpansion, was performed, in combination with bolus eptifibatide injection. Despite the lack of difference in the post-optimisation angiogram (B), thrombus and plaque prolapse diminished and minimum stent area increased.
Conclusion
Co-registration is a critical step forward for integration of OCT into PCI workflow, both pre and post procedure. Pinpoint visualisation of highly accurate lumen morphology on an angiographic monitor, combined with automated measurements, real-time 3D reconstruction and direct tableside controls may set a new standard for adoption of imaging to plan and map PCI in complex subsets.
| Impact on daily practice Co-registration of cross-sectional OCT images and angiography allows pinpoint visualisation of highly accurate lumen morphology on the angiogram. This is the next critical step to smoothen translation of a desired implantation strategy obtained by accurate OCT measurements directly into the angiographic monitor. This may potentially reduce mismatch in stent sizing and lesion coverage, ultimately leading to a better acute and long-term clinical outcome. |
Funding
The Erasmus MC and Azienda Ospedaliera Papa Giovanni XXIII have received research grants from St. Jude Medical.
Conflict of interest statement
G. Guagliumi is a consultant for Boston Scientific and St. Jude Medical. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Workflow moving image on clinical use of OCT-angiography co-registration.
Supplementary data
To read the full content of this article, please download the PDF.
Workflow moving image on clinical use of OCTangiography co-registration.

