Abstract
Background: While intravascular imaging guidance during percutaneous coronary intervention (PCI) improves outcomes, routine intravascular imaging usage remains low, in part due to perceived inefficiency and safety concerns.
Aims: The LightLab (LL) Initiative was designed to evaluate whether implementing a standardised optical coherence tomography (OCT) workflow impacts PCI safety metrics and procedural efficiency.
Methods: In this multicentre, prospective, observational study, PCI procedural data were collected over 2 years from 45 physicians at 17 US centres. OCT-guided PCI incorporating the LL workflow (N=264), a structured algorithm using routine pre- and post-PCI OCT imaging, was compared with baseline angiography-only PCI (angio) (N=428). Propensity score analysis identified 207 matched procedures. Outcomes included procedure time, radiation exposure, contrast volume, device utilisation, and treatment strategy.
Results: Compared with angiography alone, LL workflow OCT-guided PCI increased the median procedural time by 9 minutes but reduced vessel preparation time (2 min LL workflow vs 3 min angio; p<0.001) and resulted in less unplanned additional treatment (4% LL workflow vs 10% angio; p=0.01). With LL workflow OCT guidance, fewer cineangiography views were needed compared to angiography guidance, leading to decreased radiation exposure (1,133 mGy LL workflow vs 1,269 mGy angio; p=0.02), with no difference in contrast utilisation between groups (p=0.28). Furthermore, LL workflow OCT guidance resulted in fewer predilatation balloons and stents being used, more direct stent placement, and greater stent post-dilatation than angiography-guided PCI.
Conclusions: The incorporation of a standardised pre- and post-PCI OCT imaging workflow improves procedural efficiency and safety metrics, at a cost of a modestly longer procedure time.
Introduction
Intravascular imaging guidance during percutaneous coronary intervention (PCI) influences treatment decisions12, improves clinical outcomes345678, and is endorsed by society guidelines910. Despite this knowledge, routine intravascular imaging usage in clinical practice remains persistently low7. In part, the lack of adoption of intravascular imaging guidance as an adjunct to traditional angiography-guided PCI is related to preconceived notions that intravascular imaging may significantly prolong procedure times and increase contrast use or the radiation dose.
To examine the validity of these perceptions in current practice, the LightLab (LL) Initiative, a multicentre, prospective, observational study, was designed to evaluate whether implementing a standardised optical coherence tomography (OCT) workflow impacts PCI safety metrics and procedural efficiency. The LL workflow prescribes a structured algorithm to utilise the full complement of data available from high-resolution intracoronary OCT images in 2 complementary steps: 1) pre-PCI planning through assessment of plaque morphology, lesion length, and reference vessel diameter; and 2) post-PCI optimisation by evaluation for medial dissection, stent malapposition and underexpansion1112. In this study, we investigate the impact of employing a standardised OCT-guided LL workflow on PCI safety and procedural efficiency compared to angiography-guided PCI (angio) practices before introduction of the LL workflow.
Methods
Study design
The LL Initiative is a multicentre, prospective, observational study designed to evaluate the impact of implementing a standardised OCT-guided LL workflow into PCI practice using a multiphased-introduction approach (Figure 1). During the baseline study phase, operators performed 20 PCI cases per usual practice, and the baseline procedural data were utilised for comparison to subsequent study phases integrating LL protocol training by a local, embedded Field Clinical Engineer (FCE) into the clinical workflow. Propensity score matching was used as a control for differences between PCI procedures in separate study phases. Prespecified outcomes included procedure time, radiation exposure, contrast volume, device utilisation, and treatment strategy.
This study was conducted between January 2019 and March 2021 at 17 US hospitals involving a total of 45 interventional cardiologists of varying OCT imaging experience (Supplementary Figure 1). Each participating study site received institutional ethical oversight by the local institutional review board. PCI was performed at the operator’s discretion, according to current clinical standards of care, using commercially approved products, and all PCI procedures were eligible for trial inclusion. Intracoronary OCT was performed with the ILUMIEN OPTIS, OPTIS Integrated, and/or OPTIS Mobile systems with Dragonfly OPTIS or OpStar imaging catheters (all Abbott Vascular) within the approved indications for use.
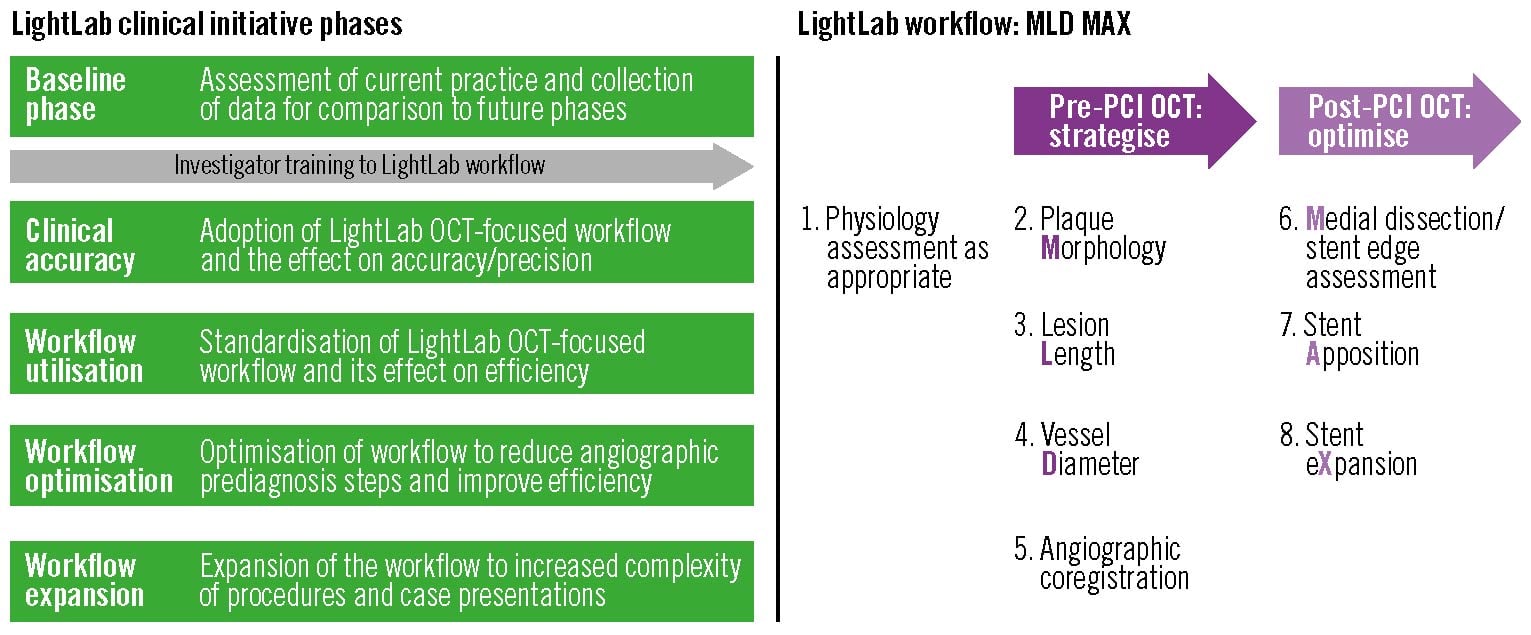
Figure 1. LightLab program phases and LightLab workflow description. LightLab programme phases and LightLab workflow description. The LightLab (LL) Initiative collected detailed procedural data from 45 interventional cardiologists at 17 US centres between January 2019 and March 2021. Following a baseline phase consisting of current clinical PCI practice, each investigator was trained on a structured OCT-guided LightLab PCI workflow algorithm that incorporated both pre- and post-PCI OCT imaging metrics. The LightLab workflow can be summarised by the acronym MLD MAX, representing: 1) pre-PCI assessment of plaque Morphology, lesion Length, and vessel Diameter; and 2) post-PCI evaluation of Medial dissection, stent Apposition, and stent eXpansion. After introducing the LightLab workflow, the investigators progressed through additional focused study phases using the LightLab workflow for PCI guidance. In this study, we compared procedural differences between LL workflow-guided PCI from the workflow optimisation phase to angiography-guided PCI from the baseline phase. OCT: optical coherence tomography; PCI: percutaneous coronary intervention
LL workflow
The LL workflow employs a structured pre- and post-PCI OCT algorithm that supplements angiography through detailed adjunctive tomographic intravascular imaging data to guide PCI planning and optimisation1112 and can be summarised by the acronym MLD MAX, where MLD stands for plaque Morphology, lesion Length, vessel Diameter and MAX for Medial dissection, stent Apposition, stent eXpansion (Figure 1). MLD is determined by pre-PCI OCT imaging, which assesses the following: 1) plaque morphology, to determine stent landing zones and the need for specialised plaque modification in calcified lesions; 2) lesion length from “normal to normal”; and 3) proximal and distal reference vessel diameters using external elastic lamina (EEL) measurements, when available, rounded down to the nearest available size to guide device selection. MAX is determined by post-PCI OCT imaging, which evaluates the following: 1) significant stent edge dissection requiring additional treatment, defined as dissection penetrating the medial layer and >1 quadrant arc; 2) zones of severe stent malapposition, defined as >3 mm length and ≥0.3 mm from the arterial wall; and 3) adequate stent expansion, to achieve a goal of >80%13.
Data collection
Data were collected independently by a field clinical engineer (FCE) on all procedures performed by study investigators in real-time onto a tablet computer using a custom data collection form (vablet) and archived maintaining Health Insurance Portability and Accountability Act (HIPAA) compliance using Azure (Microsoft). Procedures that were diagnostic only, referred for surgical revascularisation, aborted, required mechanical support, or involved a critical patient event (i.e., cardiac arrest) were excluded. In the LL workflow group, exclusions included PCI that did not include OCT imaging or did not follow the LL workflow. Consistent with the study design, no patient-specific data were collected.
Procedure and lesion characteristics
Procedure characteristics included: access site, number of lesions assessed, number of lesions treated with or without a stent, ST-elevation myocardial infarction, multivessel disease, left ventriculogram, right heart catheterisation, tortuosity, planned/staged procedure, complex lesion type, and method of closure. Lesion characteristics included: severity (Type A, B, C), presence of chronic total occlusion, in-stent restenosis, total stented length ≥28 mm, physiology assessment, calcification, ostial location, left main, involvement of bifurcation, and bypass graft interventions.
Primary endpoints
Safety metrics were evaluated according to contrast volume (cc), radiation exposure (mGy), and fluoroscopy time (minutes). The procedural efficiency was assessed by procedure duration (minutes) and device utilisation, quantified as the number of stents, balloons, and guidewires consumed per procedure. Treatment decisions were classified by the need for pre-stent vessel preparation, use of specialty balloons and atherectomy devices, stent post-dilatation, and unplanned stent implantation. As this study was designed to investigate procedural-specific clinical workflow changes, no clinical outcomes were evaluated.
Statistical analysis
Primary endpoints were evaluated according to PCI performed using the LL workflow or angiography guidance alone. To account for baseline differences, propensity scores were calculated using logistic regression by procedure matching the variables listed in Table 1 to predict the likelihood of PCI being performed using the LL workflow versus angiography only. LL workflow PCI were then matched to their closest angiography-guided PCI neighbour using their individual propensity score, with a calliper width of 0.2. Continuous and categorical variables were assessed using the Wilcoxon rank-sum test and likelihood ratio chi-squared test, respectively. A p-value <0.05 was considered significant. Due to the observational nature of the study, the sample sizes vary by output. Missing data were not imputed, so matched pairs with missing data were excluded. An unmatched analysis reports on all available results within the selected data population.
Table 1. Procedure and lesion characteristics before and after propensity matching.
| Covariate | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Angio (N=428) |
LL workflow (N=264) |
p-value | Angio (N=207) |
LL workflow (N=207) |
p-value | ||
| Lesions assessed | 1 | 59 (251/428) | 77 (203/264) | <0.0001 | 74 (154/207) | 72 (150/207) | 0.66 |
| ≥2 | 41 (177/428) | 23 (61/264) | 26 (53/207) | 28 (57/207) | |||
| Lesions treated | 1 | 72 (306/428) | 89 (234/264) | <0.0001 | 86 (177/207) | 86 (178/207) | 0.89 |
| ≥2 | 29 (122/428) | 11 (30/264) | 14 (30/207) | 14 (29/207) | |||
| Any lesions treated w/o a stent | 9 (38/428) | 5 (12/264) | 0.03 | 5 (10/207) | 4 (9/207) | 0.81 | |
| STEMI | 6 (27/428) | 5 (14/264) | 0.58 | 8 (16/207) | 7 (14/207) | 0.70 | |
| Multivessel disease | 12 (50/428) | 6 (16/264) | 0.01 | 7 (15/207) | 7 (15/207) | 1.0 | |
| Complex | 58 (249/428) | 78 (205/264) | <0.0001 | 66 (136/207) | 72 (148/207) | 0.20 | |
| Tortuosity | 5 (21/428) | 3 (8/264) | 0.22 | 4 (9/207) | 4 (8/207) | 0.80 | |
| Planned/staged | 28 (118/428) | 33 (88/264) | 0.11 | 29 (59/207) | 30 (63/207) | 0.67 | |
| Fellow present | 26 (113/428) | 34 (89/264) | 0.04 | 28 (58/207) | 29 (61/207) | 0.74 | |
| LV-gram | 32 (138/428) | 25 (66/264) | 0.04 | 28 (57/207) | 26 (54/207) | 0.74 | |
| Right heart cath | 5 (20/428) | 4 (10/264) | 0.58 | 4 (9/207) | 3 (6/207) | 0.43 | |
| Access site | radial | 45 (193/428) | 50 (132/264) | <0.0001 | 53 (110/207) | 52 (108/207) | 0.98 |
| femoral | 45 (192/428) | 48 (127/264) | 44 (91/207) | 45 (94/207) | |||
| Closure method | device | 84 (361/426) | 93 (246/264) | <0.001 | 92 (191/207) | 93 (193/207) | 0.92 |
| manual | 7 (29/428) | 5 (13/264) | 6 (13/207) | 5 (11/207) | |||
| Lesion severity | Type A | 18 (75/428) | 6 (17/264) | <0.0001 | 12 (24/207) | 8 (17/207) | 0.25 |
| Type B | 40 (171/428) | 36 (96/264) | 0.35 | 43 (88/207) | 42 (86/207) | 0.84 | |
| Type C | 39 (168/428) | 64 (168/264) | <0.0001 | 54 (112/207) | 58 (121/207) | 0.37 | |
| CTO | 11 (46/428) | 5 (13/264) | <0.01 | 9 (19/207) | 6 (13/207) | 0.27 | |
| ISR | 13 (57/428) | 20 (52/264) | 0.03 | 16 (33/207) | 17 (35/207) | 0.79 | |
| Long lesion | 44 (189/428) | 57 (151/264) | <0.001 | 50 (103/207) | 52 (108/207) | 0.62 | |
| Calcified | 22 (92/428) | 25 (65/264) | 0.34 | 24 (50/207) | 28 (58/207) | 0.37 | |
| Vein grafts | 7 (28/428) | 2 (4/264) | <0.001 | 2 (4/207) | 2 (4/207) | 1.0 | |
| Ostial lesions | 9 (37/428) | 4 (10/264 | 0.01 | 4 (8/207) | 5 (10/207) | 0.63 | |
| Left main | 4 (15/428) | 2 (6/264) | 0.35 | 2 (5/207) | 2 (5/207) | 1.0 | |
| Bifurcation | 3 (12/428) | 0 (1/264) | 0.01 | 0 (0/207) | 0 (1/207) | 0.24 | |
| Invasive physiology assessment | 9 (37/428) | 12 (31/264) | 0.19 | 10 (21/207) | 12 (25/207) | 0.53 | |
| Values are given as % (n/N). Angio: angiography-guided PCI alone; CTO: chronic total occlusion; ISR: in-stent restenosis; LL: LightLab; LL workflow: OCT-guided PCI with the LL workflow; LV-gram: left ventriculogram; STEMI: ST-elevation myocardial infarction | |||||||
Results
Over a 2-year period, data were gathered from 3,526 invasive procedures in the baseline and LL workflow optimisation phases (Figure 2). Of these, 2,078 (58.9%) were excluded as only diagnostic angiography was performed. For the remaining procedures, out of 942 baseline phase PCI, 428 (45.4%) PCI guided by angiography alone met all the inclusion criteria, with the most common exclusion criteria being OCT or intravascular ultrasound (IVUS) use (52.9%). The LL workflow optimisation phase had 476 PCI of which 264 (55.5%) met the inclusion criteria, with 25.2% of excluded PCI due to non-compliance with the LL workflow. After propensity score analysis, 221 baseline phase angiography-guided PCI and 57 LL workflow OCT-guided PCI were unmatched and excluded, leaving a total of 207 propensity-matched pairs for comparison. For comparison, a secondary analysis of 325 propensity-matched procedures was performed between baseline angiography-guided PCI and baseline variable workflow OCT-guided PCI (Supplementary Figure 2, Supplementary Table 1).
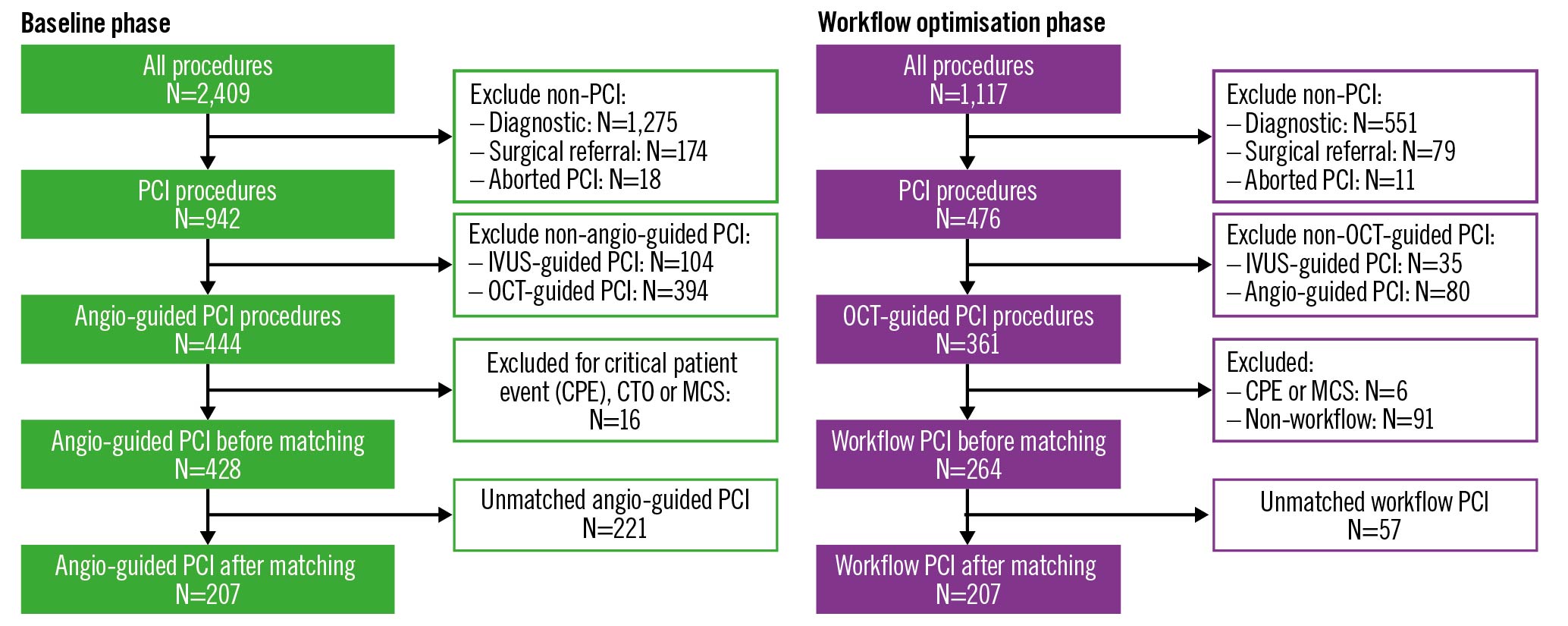
Figure 2. Study flowchart for angiography-guided and LightLab (LL) workflow OCT-guided PCI. Angiography-guided PCI procedures from the baseline phase were compared to OCT-guided PCI procedures using the LL workflow from the workflow optimisation phase. After exclusions, each group was matched based on propensity scores in order to control for differences between PCI procedures in the separate study phases. CTO: chronic total occlusion; IVUS: intravascular ultrasound; MCS: mechanical circulatory support; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Procedural and lesion characteristics
A summary of the detailed procedure and lesion characteristics for the angiography alone and LL workflow PCI groups before and after propensity matching are shown in Table 1. Overall, the majority of PCI in both groups were for complex, single-vessel disease of ACC/AHA type B/C lesions. Long lesions of ≥28 mm were heavily represented in both groups (57% LL workflow vs 44% angio), with calcified vessels present at nearly equal frequency (25% LL workflow vs 22% angio). Vein graft, left main, ostial lesion location, and bifurcation interventions accounted for approximately 8% of LL workflow and 21% of angiography-guided PCI. Invasive physiology assessment was used sparingly in both groups (12% LL workflow vs 9% angio). After propensity matching, no significant differences were observed between the angiography-guided and LL workflow OCT-guided PCI populations (Table 1).
Safety of implementing the LL workflow
The safety implications of introducing the standardised OCT-guided LL workflow during PCI were assessed by comparing the procedural contrast volume, radiation exposure, and fluoroscopy time to the baseline phase angiography-only PCI data (Figure 3). On average, a total of 2 OCT pullbacks (i.e., 1 pre- and 1 post-PCI) were performed in 78% of LL workflow cases, with 3 OCT pullbacks in 17%, and 4 or 5 (maximum observed) occurring in 5% of procedures. While the fluoroscopy time was similar, with the OCT-guided LL workflow fewer diagnostic cineangiography views were required compared to angiography alone (6 LL workflow vs 7 angio; p<0.01). Less cineangiography translated into a reduction in total radiation exposure when the LL workflow was used compared to angiography-guided PCI (1,133 mGy LL workflow vs 1,269 mGy angio; p=0.02), with no significant difference in contrast volume usage (150 cc LL workflow vs 146 cc angio; p=0.28). In comparison, variable workflow OCT-guided PCI (pre- and post-PCI OCT performed in 46% of procedures) (Supplementary Table 2) demonstrated no difference in the number of cineangiographic views obtained compared to angiography alone, with associated greater radiation and contrast usage (Supplementary Table 3).
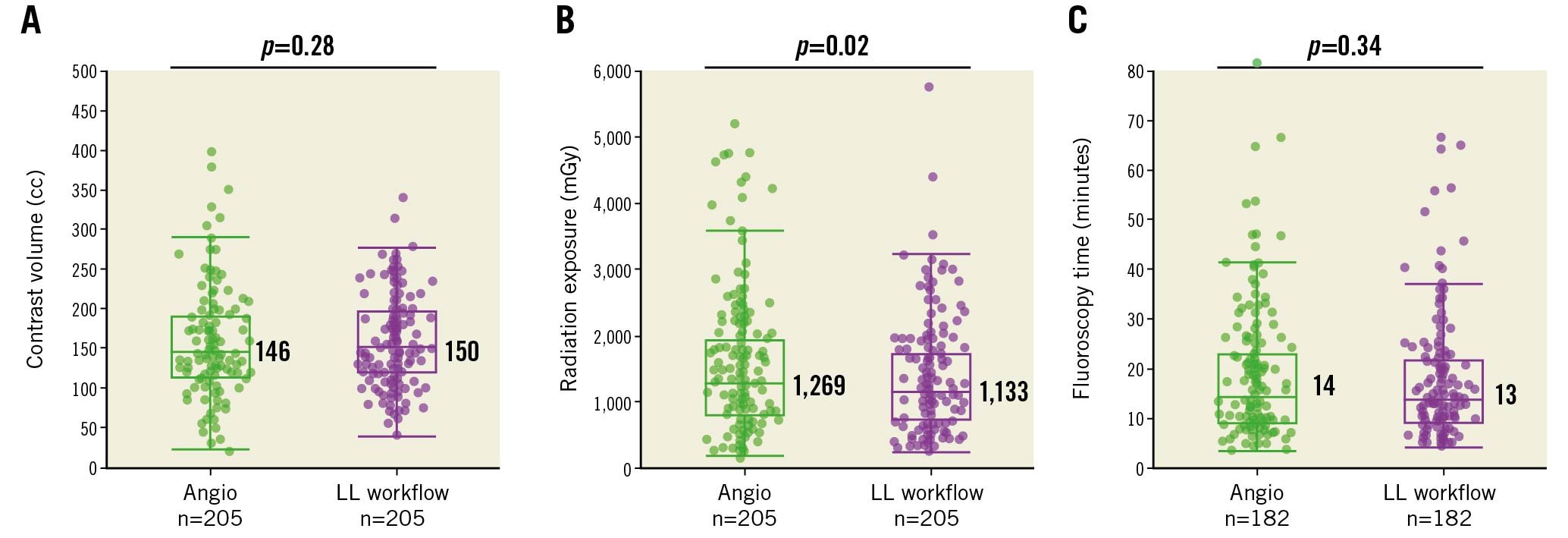
Figure 3. Comparison of safety metrics between angiography-guided and standardised workflow OCT-guided PCI. Scatter box plots for (A) contrast volume, (B) radiation exposure, and (C) fluoroscopy time between propensity-matched PCI procedures that utilised angiography guidance alone (green) or the LightLab workflow OCT guidance (purple). The median values are labelled, and the whiskers represent Q1-1.5*IQR and Q3+1.5*IQR, where Q1=first quartile, Q3=third quartile, and IQR=interquartile range, or the outermost data point (excluding outliers) if the calculated values exceed the upper or lower data values. Angio: angiography-guided PCI; LL: LightLab; LL workflow: LL workflow OCT-guided PCI; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
INFLUEnce of the LL workflow on procedural efficiency
When compared to angiography-guided PCI alone, the introduction of the LL workflow influenced multiple procedural efficiency metrics. Overall, the procedure time was longer in the LL workflow group by 9 minutes (45 min LL workflow vs 36 min angio; p<0.0001) (Figure 4). While pre-OCT procedure planning and post-OCT stent optimisation accounted for a duration of approximately 10 min, procedural time savings were observed using the LL workflow in both the transition from the diagnostic to the intervention phase (6 min LL workflow vs 7 min angio; p<0.001) and the vessel preparation prior to stent implantation (2 min LL workflow vs 3 min angio; p<0.001). In addition to the procedure time, PCI using the LL workflow altered device utilisation compared to angiography-guided PCI alone (Table 2). Introduction of the LL workflow resulted in significantly fewer compliant balloons (p<0.0001) and stents (p=0.0478) required per procedure over angiography guidance alone, with a substantial increase in non-compliant balloon use (p<0.0001). When variable workflow OCT was used, the procedure times were longer (Supplementary Table 4) and there was no difference in the number of stents used per group (Supplementary Table 5); however, the number of non-compliant balloons remained greater than with angiography-guided PCI alone.
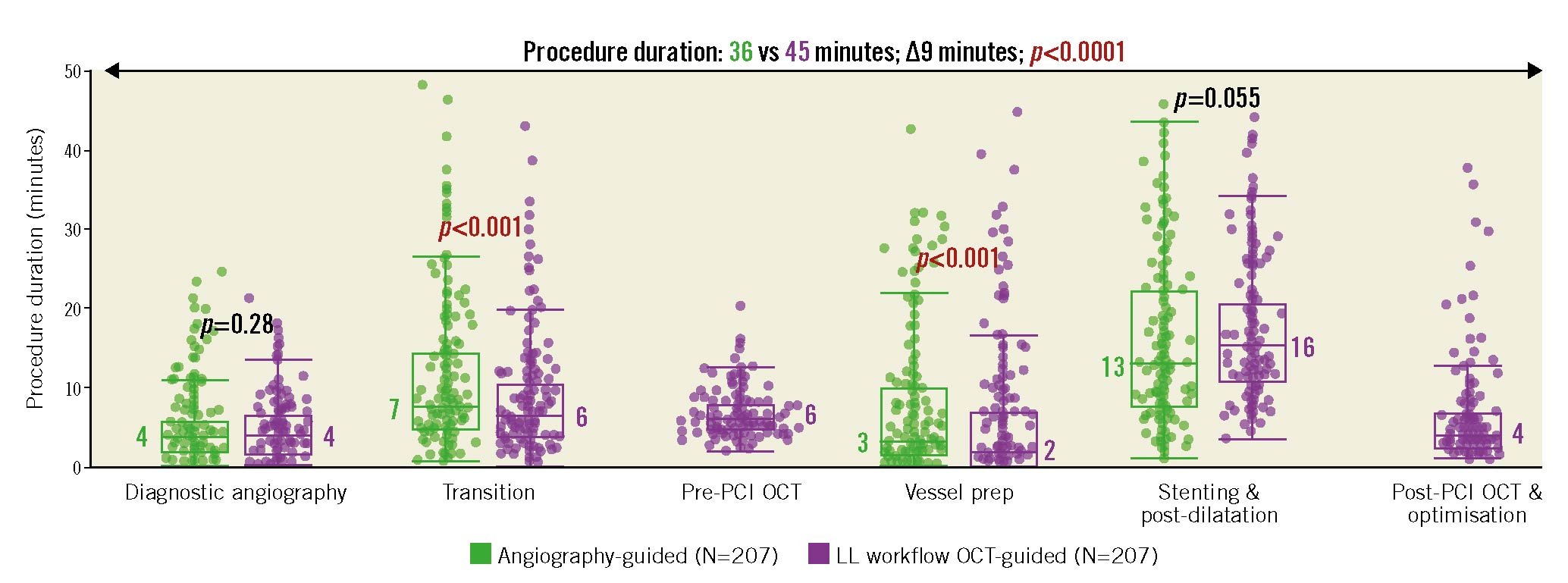
Figure 4. Procedure durations after introducing a standardised OCT workflow compared to angiography alone. Box plots representing the different measured time durations spent on each component of the procedure for propensity-matched angiography-guided or LightLab (LL) workflow OCT-guided PCI. Following diagnostic angiography, the transition period accounts for the time until the guidewire crosses the lesion. The median values are labelled, and the whiskers represent Q1-1.5*IQR and Q3+1.5*IQR, where Q1=first quartile, Q3=third quartile, and IQR=interquartile range, or the outermost data point (excluding outliers) if the calculated values exceed the upper or lower data values. OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Table 2. Device utilisation changes after implementation of the standardised OCT-guided PCI workflow.
| Product utilisation | Angio (N=207) | LL workflow (N=207) | p-value |
|---|---|---|---|
| Stents used | 1.5±0.86 | 1.3±0.67 | 0.0478 |
| Non-compliant balloons used | 1.3±1.13 | 1.9±1.19 | <0.0001 |
| Compliant balloons used | 1.2±0.87 | 0.8±0.74 | <0.0001 |
| Guidewires used | 1.6±1.12 | 1.4±0.72 | <0.01 |
| Values are mean±SD. Angio: angiography-guided PCI alone; LL: LightLab; LL workflow: LL workflow OCT-guided PCI; OCT: optical coherence tomography; PCI: percutaneous coronary intervention | |||
Impact of the LL workflow on treatment decisions
To investigate the impact of the LL workflow on PCI treatment decisions, data were analysed on the use of pre-stent vessel preparation, specialty balloons and atherectomy devices, stent post-dilatation, and unplanned stent implantation. The use of pre-OCT imaging information resulted in more lesions in the LL workflow group being treated without vessel preparation prior to stent implantation (25% LL workflow vs 11% angio; p<0.0001) (Figure 5). In addition, LL workflow PCI were more likely to employ specialty balloons (cutting/scoring) or atherectomy for plaque modification prior to stent placement (20% LL workflow vs 13% angio; p=0.049) (Supplementary Table 6). After deploying the stent, a significant increase in stent post-dilatation was observed with the LL workflow (96% LL workflow vs 60% angio, p<0.0001) (Table 3), which occurred prior to the final OCT in 90% of cases. Despite the high prevalence of post-dilatation in the LL workflow group, the final OCT imaging results still led to additional stent optimisation with supplementary post-dilatation being performed 35% of the time (Supplementary Table 7). In the LL workflow OCT-guided PCI group, operators achieved >80% stent expansion in the majority of cases (% expansion, median [IQR]: 85% [76%-95%]). While post-dilatation and stent optimisation was greater in the LL workflow group, overall there were fewer lesions that required unplanned additional treatment compared to angiography-guided PCI alone (4% LL workflow vs 10% angio; p=0.01).
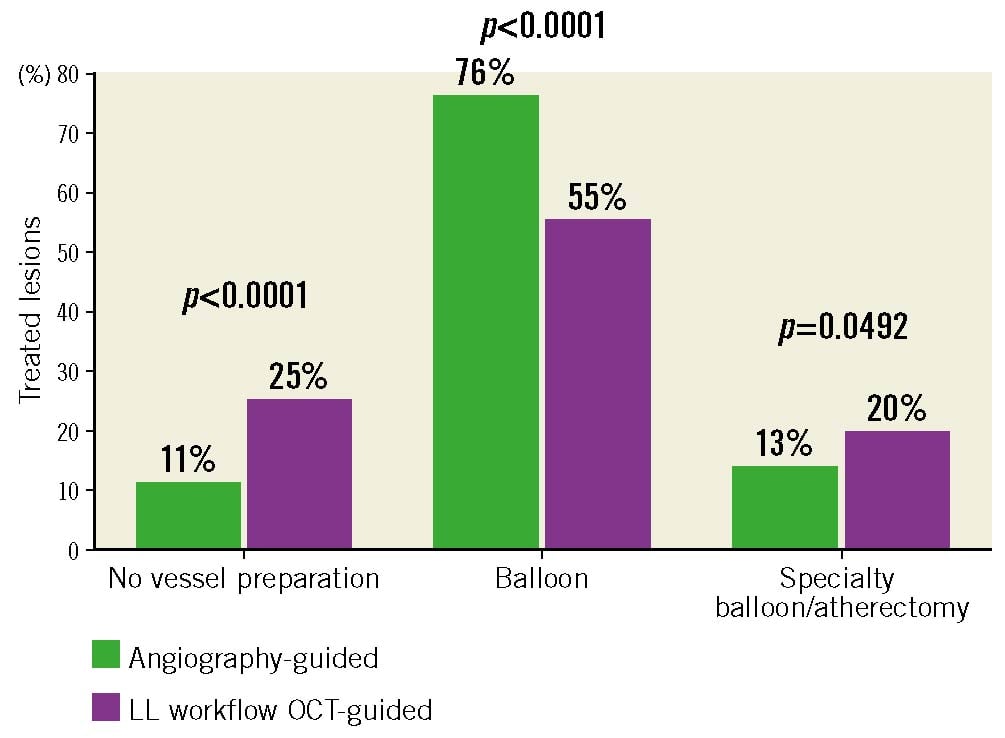
Figure 5. Lesion treatment decisions in angiography-guided and LightLab (LL) workflow OCT-guided PCI procedures. The percentage of total lesions treated with different interventional strategies in the propensity-matched angiography-guided and LightLab workflow OCT-guided PCI groups. No vessel preparation: indicates direct stent placement without prior balloon inflation or plaque modification. Specialty balloon: represents a combination of scoring or cutting balloons. OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Table 3. Treatment decisions in angiography-guided and standardised workflow OCT-guided PCI procedures.
| Lesion outputs | Angio (N=207; L=239) | LL workflow (N=207; L=240) | p-value |
|---|---|---|---|
| Lesions treated with unplanned additional stent | 10 (24/231) | 4 (10/235) | 0.01 |
| Lesions with post-dilatation | 60 (138/231) | 96 (218/227) | <0.0001 |
| Post-dilatation performed before post-PCI OCT |
– | 90 (206/228) | – |
| Optimisation after initial post-dilatation | – | 35 (72/206) | – |
| Lesions with vessel preparation | 89 (212/238) | 75 (179/239) | <0.0001 |
| Values are given as % (n/N). Angio: angiography-guided PCI alone; L: lesion; LL workflow: LightLab workflow OCT-guided PCI; OCT: optical coherence tomography; PCI: percutaneous coronary intervention | |||
Discussion
Intravascular imaging has emerged as a key adjunct which positively impacts decision-making and improves PCI outcomes compared to the traditional PCI approach, which relies on angiography guidance alone12345678. The growth of data supporting intravascular imaging to supplement angiography during PCI has recently culminated in updated ACC/AHA/SCAI joint committee practice guidelines endorsing intravascular imaging-guided PCI10. However, a disconnect continues to exist between the current knowledge base regarding the known benefits of intravascular imaging and the sparse real-world integration of intravascular imaging-guided PCI into modern day US clinical practice7. While intravascular imaging uptake has been hindered, in part, by theoretical safety concerns and potential time inefficiencies, studies demonstrate that there remains a general lack of proper training and familiarity with intravascular imaging criteria for stent optimisation14, with the end result being variable practice patterns that lessen the derived clinical benefits of an intravascular imaging-guided PCI strategy. Even among clinical trials, different criteria for optimal stent implantation have been utilised25681215, with inconsistent pre-PCI imaging evaluation, further contributing to a lack of consensus as to the most beneficial intravascular imaging PCI workflow approach.
To evaluate the barriers to intravascular imaging adoption in clinical practice, the LightLab Initiative, a multicentre, prospective, observational study, was designed to investigate whether implementing a standardised OCT intravascular imaging workflow impacts PCI safety metrics and procedural efficiency. Compared to procedures using angiography guidance alone, the main findings of our study indicate that OCT-guided PCI employing the LL workflow: 1) improves procedural safety by decreasing the radiation dose with no difference in contrast utilisation; 2) enhances procedural efficiency by decreasing the need for universal vessel preparation in select lesions, while increasing the frequency of specialty balloon, atherectomy, and post-dilatation to optimise stent implantation; and 3) reduces product utilisation by improving device selection based on precise sizing and identifying appropriate stent landing zones that lessen the likelihood that additional vessel treatment will be required. Overall, supporting prior work with structured pre- and post-imaging algorithms in complex lesion subsets16, the benefits of using a standardised LL workflow during OCT-guided PCI has the potential to result in greater adoption of routine intravascular imaging PCI use.
OCT-guided PCI and the MLD MAX LightLab workflow
OCT enables rapid acquisition (up to 40 mm/sec) of high spatial resolution (10-20 μm) intravascular tomographic images that can be precisely coregistered with the angiogram for procedural reference11. In this way, OCT offers a comprehensive assessment of multiple vessel and stent structural parameters that have been demonstrated to influence procedural decision-making and improve clinical outcomes16151718. The clinical benefits of OCT-guided PCI are maximised when specific intravascular imaging criteria are achieved, including adequate stent expansion, lack of significant edge dissection or residual untreated disease at the stent edges15681217. The LL workflow incorporates these key intravascular imaging metrics into OCT-guided PCI, as specified by the MLD MAX algorithm1112. Adhering to the MLD MAX LL workflow allows interventional operators to exploit the full complement of available OCT imaging data in 2 complementary steps performed before and after stent implantation.
The first phase of MLD MAX involves pre-PCI OCT imaging to determine plaque morphology, lesion length, and vessel diameter (MLD). By precisely understanding true vessel size (based on external elastic lamina [EEL] measures when possible) and disease length (stenting from “normal-to-normal” vessel segments), operators are able to select the specific balloon and stent sizes in advance, improving procedural efficiency and eliminating subjective visual size estimations that may lead to suboptimal stent results. Plaque morphology assessment further allows a priori determination of the presence of significant calcification that may preclude optimal stent expansion and require advanced plaque modification, such as cutting/scoring balloons, lithotripsy, or atherectomy. To assist with these determinations, intravascular imaging calcium scoring systems have been developed1920. In the near term, artificial intelligence software features that can determine calcium arc, thickness, and length are likely to further enhance the speed and accuracy of point-of-care OCT image interpretation21.
Following stent implantation, the post-PCI OCT phase of MLD MAX interrogates the quality of the stent result and evaluates for clinically significant stent-related complications11. Medial dissection, a predictor of stent failure that is particularly concerning when present at the distal stent edge18, is assessed at the proximal and distal stent edges and, when indicated, is typically corrected with additional stent placement. Stent malapposition and expansion (MAX) are then interrogated sequentially, and, when discovered, are treated most often with further high-pressure balloon post-dilatation to achieve the recommended goal of >80% stent expansion13. Preventing stent underexpansion through proper lesion preparation and stent optimisation is critical for optimal long-term results, as inadequate stent expansion is the most important predictor of subsequent stent failure due to stent thrombosis or restenosis22.
Implementing the LL workflow improves PCI safety and procedural efficiency
With a minimal time investment, our study demonstrates that implementing the structured LL workflow pre- and post-PCI OCT imaging algorithm offers several procedural safety and efficiency advantages compared to PCI performed with angiography guidance alone (Central illustration). Compared to previous OCT and IVUS trials where intravascular imaging added 15-20 min over angiography alone121520, the results of which are supported by our current data where variable workflow OCT procedures were similarly longer, the 9 min additional procedure time using the OCT-guided LL workflow represents a time saving of up to 50%. From a safety perspective, the detailed tomographic information provided by intravascular OCT diminishes the reliance on angiography, resulting in fewer cineangiography runs and less overall case radiation to the patient and catheterisation laboratory staff when the structured LL workflow is utilised. With the advent of OCT-based angiographic coregistration systems, operators are able to obtain simultaneous intravascular and angiographic images, further reducing supplementary angiographic views to resolve areas of vessel overlap, foreshortening, and tortuosity. In addition, OCT with coregistered angiography improves operator confidence in localising stent placement and identifying specific anatomic regions of interest that may need additional or specialised treatment. Notably, unlike what was observed in our variable workflow OCT analysis, which aligns with prior OCT and IVUS studies where a contrast penalty was observed when using intravascular imaging121520, the safety benefits achieved with the LL workflow OCT-guided PCI were possible without an increase in total contrast use compared to angiography alone. Thus, the structured LL workflow algorithm can be safely implemented during PCI without concern for increasing the risk of contrast nephropathy. Overall, our findings suggest that, with the advent of modern-day coregistered angiography and OCT, implementing the LL workflow for OCT guidance can improve procedural safety over angiography-guided PCI alone by decreasing the radiation dose with no impact on contrast utilisation.
In addition to safety benefits, PCI procedural efficiency was improved with the OCT-guided LL workflow compared to angiography guidance alone. The information obtained from pre-PCI OCT imaging was able to individualise treatment decisions based on the observed plaque morphology, which is of particular importance when substantial calcification is present and more aggressive plaque modification is required. In addition, pre-PCI OCT imaging facilitates choosing appropriate size balloons and stents in advance of the intervention, which led to fewer longer stents being used per procedure when the LL workflow was implemented, as well as a substantial increase in stent post-dilatation and optimisation, resulting in the majority of stents in the LL workflow group achieving the clinically recommended >80% expansion. As stent underexpansion remains the most significant risk factor for subsequent stent failure22, and intravascular imaging-guided post-dilatation is superior for stent expansion and clinical outcomes compared to angiography alone23, OCT-guided stent optimisation is key to mitigate this future risk. Although this would require a further dedicated cost-effectiveness study beyond the scope of this work, the observed safety and procedural efficiency benefits associated with the OCT-guided LL workflow may also lead to cost savings during the procedure due to more parsimonious device selection (e.g., fewer stents) that offset the OCT catheter cost, with downstream economic advantages related to less target vessel revascularisation, as demonstrated in randomised controlled trials58. The results of the ongoing ILUMIEN IV randomised clinical trial (ClinicalTrials.gov: NCT03507777) comparing OCT-guided PCI to angiography guidance alone will provide further critical data on the significance of the LL workflow on patient outcomes24.
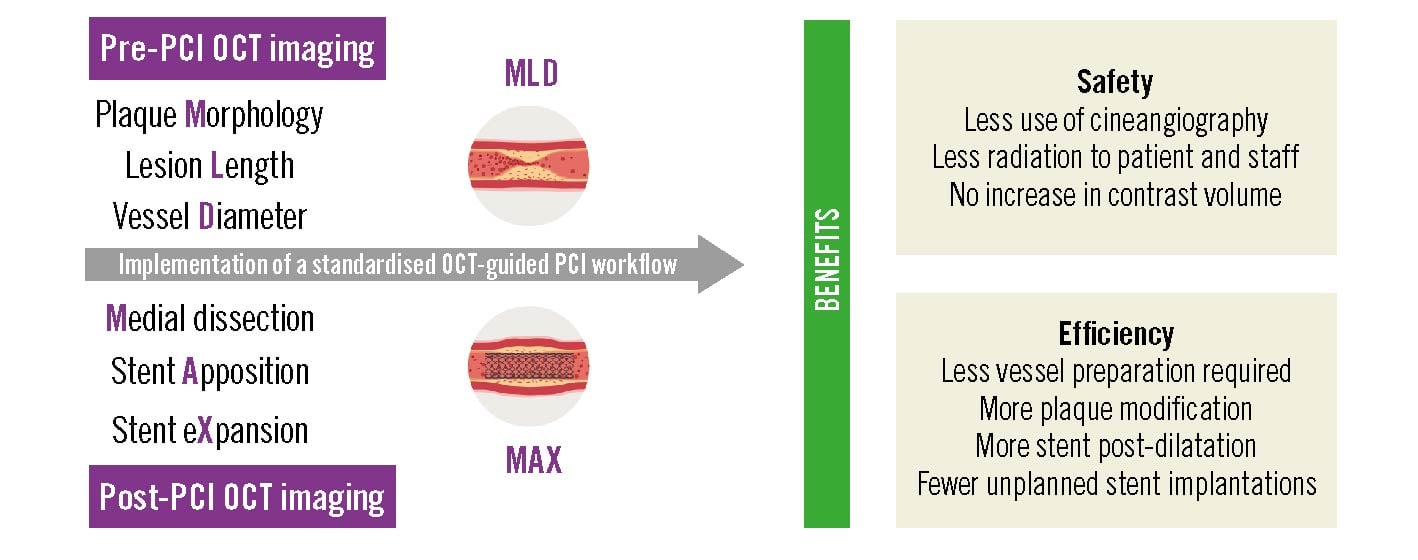
Central illustration. Impact of a standardised OCT-guided PCI workflow on procedural safety and efficacy. Clinical safety and procedural efficiency benefits are evident with a standardised pre- and post-PCI OCT imaging workflow that optimises coronary stent implantation. OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Limitations
The results of this work are limited due to the observational study design and the fact that only OCT-guided procedures where the prescribed LL workflow could be completed in all treated lesions were included. Some of the derived safety and efficiency improvements using the LL workflow may be related to: 1) providing operators with an algorithmic structure that formalises the required steps during PCI, 2) improvements in LL workflow operator proficiency during the study leading to higher-quality OCT image data collection with fewer repeated OCT pullbacks needed, and 3) greater comfort with OCT guidance, lessening reliance on multiple angiographic views. Additional factors impacting radiation dose, such as the patient’s size and fluoroscopy equipment, were not able to be accounted for; and, furthermore, it is unclear if the observed reductions in radiation exposure would translate into clinically meaningful safety events. There are also limitations as to the generalisability of the results, since the study was performed amongst 45 interventional operators in catheterisation laboratories that already had OCT imaging system capabilities, and the majority of treated lesions were complex. Furthermore, longitudinal operator and staff training by an FCE, as performed in this study, is not necessarily feasible for all institutions planning to incorporate the LL workflow into OCT-guided PCI clinical practice.
Conclusions
The incorporation of a standardised OCT workflow to guide PCI improves procedural efficiency and safety metrics. As intravascular imaging improves PCI outcomes and is endorsed by society guidelines, these data support greater adoption of routine OCT use in clinical practice.
Impact on daily practice
Intravascular imaging guidance during percutaneous coronary intervention (PCI) influences treatment decisions and improves clinical outcomes; however, intravascular imaging use during PCI remains low, in part, due to perceived safety and efficiency concerns. The LightLab (LL) Initiative evaluated whether a structured optical coherence tomography (OCT) imaging workflow impacts PCI safety and procedural efficiency metrics. Compared to PCI performed by angiography guidance alone, implementing an OCT-guided LL workflow decreased radiation exposure with no difference in contrast utilisation and enhanced procedural efficiency by guiding proper device selection, leading to less product utilisation and fewer unplanned treatments. Outcome data from the ongoing ILUMIEN IV trial will further enhance our understanding of the clinical benefits of using the LL workflow approach.
Acknowledgements
This work would not be possible without the dedication of all participating LightLab physician champions and their support staff, as well as data collection by the LightLab field clinical team.
Funding
The LightLab Initiative is a clinical programme funded by Abbott Vascular.
Conflict of interest statement
E.A. Osborn is a consultant for Abbott Vascular, Canon, OpSens, and Philips; has undertaken sponsored research from Dyad Medical, NuPulseCV, and OpSens; is a member of the scientific advisory board of Dyad Medical and holds equity in this company. F.J. Zidar is a consultant and speaker for Abbott Vascular. E.C. Korngold is a consultant and speaker for Abbott Vascular, Boston Scientific, CR Bard, and Medtronic. J. Rauch is on the speakers bureau for Abbott Vascular. J. Buccola, H. Garcia Cabrera, R.J. Rapoza, and N.E.J. West are employees of Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

