Drug-eluting stents (DES) have been in the armoury of interventional cardiologists for over a decade1 and much water has passed under the bridge. Whilst their efficacy has justifiably never been questioned, their safety has rarely moved off the front page2-4. Reassuringly, contemporary randomised data assessing the performance of newer-generation DES in all-comers’ populations5-7, and the long-awaited results from the PROTECT study8, the only randomised study powered for stent thrombosis, have allowed us to virtually extinguish the firestorm that was first ignited back in 2006.
Despite these accomplishments, the need for newer DES remains, not least because stent thrombosis and delayed restenosis have been far from eliminated. Consequently the new iterations of DES strive to improve vascular healing, whilst still inhibiting the physiological repair processes common to any foreign implant9. No component of these devices has been left untouched, with modifications directed at the composition and permanence of the stent platform; the thickness and shape of the stent struts; the arrangement of the stent cells; the location, permanence and biocompatibility of the stent polymer; and the dose and type of antiproliferative agent eluted from the device10. All permutations are driven by the desire to develop the ultimate device – i.e., one that offers long-term efficacy and safety.
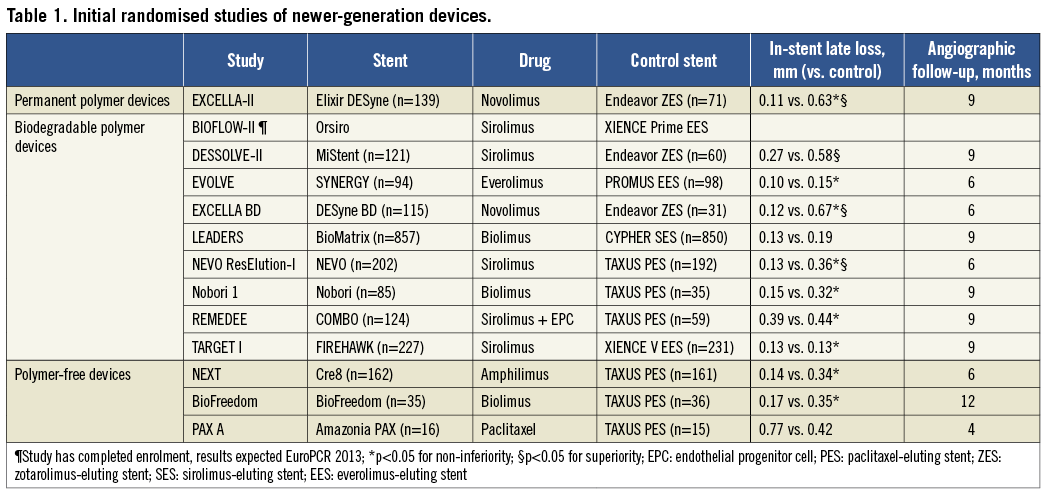
In the present issue of EuroIntervention, Gao et al report the results of the TARGET I study, the first randomised trial of the FIREHAWK® stent (MicroPort Medical, Shanghai, China), a sirolimus-eluting stent (SES) with a cobalt-chromium platform and a novel abluminal groove-filled biodegradable poly-lactic acid polymer11. The study was powered for late lumen loss at nine-month follow-up and, as expected during the early assessment of a new device, enrolled a low-risk population. Results confirmed the non-inferiority of the FIREHAWK stent compared to XIENCE V® everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA) with respect to late loss. The clinical event rates were low, which is reflective of the study population and provides little information on the stent’s performance. The investigators must be congratulated for a well-conducted study, with high rates of procedural success and angiographic follow-up. In addition, they must be given credit for the conviction they showed in their device by selecting the market leader as their choice of a competitor stent. There is no guidance from regulatory authorities on the choice of the control stent; however, new DES are commonly pitched against weaker, less efficacious DES (Table 1), such as the Endeavor® zotarolimus-eluting stent (E-ZES; Medtronic Cardiovascular, Santa Rosa, CA, USA) or the TAXUS™ paclitaxel-eluting stent (PES; Boston Scientific, Natick, MA, USA)9. This all but guarantees non-inferiority, but does not always generate the necessary confidence in the stent.
Many aspects of the FIREHAWK stent’s design are driven by a desire to minimise stent thrombosis, and these merit further scrutiny.
Reservoir technology
The use of a reservoir on a stent has been tried before12-14 and remains an attractive strategy for combatting stent thrombosis as it minimises vessel wall inflammation through a reduction in the spatial and temporal interaction between polymer, drug and vessel wall. Historical attempts at this design have failed to progress despite early promise12,13, although it must be appreciated that this has had little do with the reservoirs and more so with other factors. The demise of the CoStar™ stent (Conor Medsystems, Menlo Park, CA, USA) was driven by high rates of repeat revascularisation compared to PES in the CoStar II study, which was attributable to an insufficient dose of paclitaxel and a detrimental alteration to its release kinetics12. More recently, the sirolimus-eluting NEVO™ stent (Cordis Corp., Johnson & Johnson, Inc., Warren, NJ, USA) was withdrawn during its pivotal all-comers trial due to problems with its balloon catheter rather than any concerns over safety or efficacy. A major difference in reservoir design exists between these defunct stents, which used a laser to create a reservoir that extended across the full thickness of a strut, and contemporary reservoir DES such as the Cre8™ (CID Vascular, Saluggia, Italy) and the FIREHAWK which use grooves that are scored on the outer surface of the strut, and are therefore only of partial thickness. Irrespective of the depth of the reservoir, the linkage of cells on these stents is achieved through the use of flexible sinusoidal bridges, which take the brunt of the expansile forces generated by balloon inflation. This subtle design feature ensures that stent deformation is confined to only 10% of the stent; hence the struts are mere bystanders during stent expansion, preserving the integrity of the reservoir’s contents. Despite these favourable features, other than the encouraging early data from the NEXT study of the Cre8 stent14 which elutes a macrocyclic lactone in the absence of a polymer from its reservoirs, there is little proof of concept. The single-arm 1,000 all-comer patient pARTicip8 study of the Cre8 stent has now completed enrolment and will provide vital data which will fuel any further development of DES with reservoirs.
Sirolimus
The use of sirolimus, a highly regarded potent antiproliferative agent with a proven track record, is wise, especially considering that the CYPHER™ SES (Cordis Corp., Johnson & Johnson, Inc., Warren, NJ, USA), which is sadly no longer with us, left the DES arena whilst still being a very difficult competitor to beat15. The low late loss in the TARGET I study (0.13±0.24 mm) reflects sirolimus’s efficacy, and this is achieved despite its dose being reduced to approximately a third of that present on the Cypher SES; importantly, release kinetics remain comparable (unpublished data). This latter point is significant in the light of data from Serruys et al which demonstrate that optimal drug kinetics are as important in inhibiting neointimal hyperplasia as absolute drug dose16. It is noteworthy that the designers of the NEVO stent learned from the failures of the CoStar stent and used exactly the same dose and release kinetics of sirolimus as on the Cypher SES. The same cannot be said of the FIREHAWK and only after evaluation in more complex lesions will a conclusion be possible on the appropriateness of this decision.
Biodegradable polymer
The use of a biodegradable polymer on the FIREHAWK is aimed at improving stent safety. The theory behind this approach is well documented, and the technology is used in many contemporary DES to elute a variety of different macrocylic lactone drugs9. Data from the LEADERS study provide support for the concept, with significantly lower rates of very late stent thrombosis being observed in patients receiving a biodegradable polymer DES compared to a permanent polymer DES17. It must be acknowledged that the LEADERS study was not powered for stent thrombosis, so these observations may be purely speculative and certainly require reassessment. Undoubtedly, together with safety, it is important to ensure that use of a biodegradable polymer does not adversely affect efficacy. Analysis of biodegradable polymers after stent expansion with the use of electron microscopy has raised the possibility of polymer cracking, which could conceivably reduce efficacy18. Reassuringly, data from the ISAR-TEST 3 confirm comparable short-term angiographic and clinical outcomes irrespective of whether sirolimus was eluted by a permanent or biodegradable polymer19. Nevertheless, polymer disruption and any potential clinical sequelae are minimised with the FIREHAWK stent owing to its design and the diversion of inflation balloon expansile forces away from the struts.
Report card
So what can we conclude about the FIREHAWK stent?
Certainly it has a design that favours efficacy and safety; however, the proof of these can only come from clinical data, and currently these are not robust enough to make any definitive conclusions. Thus far, the FIREHAWK stent has done what was asked of it; however, a pivotal multicentre randomised all-comers study is required, not only as this is a necessity for regulatory approval, but also to gain the confidence of interventionalists (and patients). The year-end report card for the FIREHAWK should therefore read, “Excellent potential; promising start; in need of a greater challenge; hopefully won’t disappoint in later years.”
Conflict of interest statement
The author has no conflicts of interest to declare.

