![]()
Abstract
Aims: The aim of this study was to examine the effect of the daughter branches on the haemodynamics and the potential prediction of atherosclerotic plaque development as well as the best flow division model for accurate blood flow modelling.
Methods and results: We analysed computed tomography coronary angiography retrospective data portraying 17 coronary artery bifurcations in 15 patients recruited into the PROSPECT MSCT study. Baseline and three-year follow-up imaging data were used to reconstruct coronary artery anatomy. In the baseline models blood flow simulations were performed using three flow division approaches: stress-free, Murray’s law and Doriot’s fit. Blood flow simulation was also performed omitting the daughter branch. The association between ESS estimated in models that incorporated the daughter branches and lumen reduction was higher than the cases where the side branch was omitted. Murray’s law provides the most accurate results when comparing the different flow division models. More specifically, low ESS is a predictor of significant lumen reduction (p=0.007), plaque burden increase (p=0.0006) and necrotic core change (p=0.025).
Conclusions: The ESS distribution in coronary models including the daughter branches and based on the calculations implementing Murray’s law allows more accurate prediction of atherosclerotic evolution than ESS estimated in models including only the main vessel.
Abbreviations
3D: three-dimensional
AHA: American Heart Association
CTCA: computed tomography coronary angiography
ESS: endothelial shear stress
LAD: left anterior descending
LCX: left circumflex artery
RCA: right coronary artery
Introduction
Cardiovascular disease is one of the most common causes of death regulated by systemic factors but also presenting focal manifestations1. Atherosclerotic lesions are seen more often in segments with flow disturbances and low endothelial shear stress (ESS), such as in coronary bifurcations and in the inner side of curved vessels, while low ESS is associated with atherosclerotic disease progression and future adverse cardiovascular events2,3. Coronary reconstruction in these studies was restricted to the main vessel and did not include the side branches, thus it was not possible to assess the effect of flow division on atherosclerotic evolution. Recently, van der Giessen et al developed a methodology for reconstruction of the main vessel and daughter branches by fusing intravascular and angiographic imaging data4. It was demonstrated in 21 patients that the ESS distribution differed in segments in which they incorporated side branches from those in which they did not incorporate the side branches5. Reports have shown that computed tomography coronary angiography (CTCA) appears able to assess the extent and severity of coronary atherosclerosis and estimate ESS distribution accurately6,7. Nevertheless, the value of complex coronary modelling that includes side branches in estimating the ESS and predicting atherosclerotic evolution more accurately is as yet unclear.
The aim of the present study was to analyse serial CTCA imaging data that portray coronary bifurcations, examine whether ESS can predict disease progression more accurately when daughter branches are incorporated in coronary modelling and blood flow simulation, and investigate which model of flow division in coronary bifurcations allows the most accurate prediction of atherosclerotic evolution.
Methods
The rationale of the study design was to perform 3D reconstruction of the coronary bifurcations of the selected population. The aim was then to perform blood flow modelling according to the proposed flow division models and boundary conditions, and finally to perform statistical analysis between the simulation results, baseline characteristics and disease progression, as this is estimated between the follow-up and baseline examinations. The design of the study is shown in Figure 1.
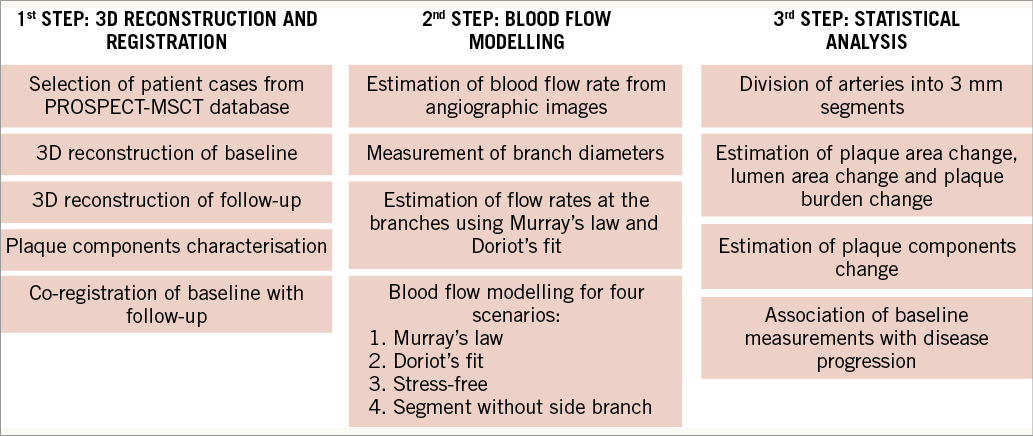
Figure 1. Study design. Initially, CTCA data from the PROSPECT-MSCT database were acquired and used for 3D reconstruction. Then blood flow modelling was performed and, finally, statistical analysis was carried out to identify predictors of disease progression.
STUDIED POPULATION AND IMAGE ACQUISITION
Retrospective data from the patients recruited into the PROSPECT-MSCT study were analysed. The design and inclusion criteria of the study have been previously described8. In brief, this study included patients admitted with an acute coronary syndrome who had complete revascularisation followed by IVUS imaging in the non-culprit vessels and then CTCA at baseline and at three-year follow-up. Imaging at baseline was performed using a 64-slice CTCA scanner (SOMATOM Sensation 64; Siemens Medical Solutions, Forchheim, Germany), while at follow-up a 64-slice dual-source CT scanner (SOMATOM Definition; Siemens Medical Solutions) was used. The acquisition protocol has been presented previously9. In total, 36 patients underwent the follow-up protocol, while 58 arteries were finally reconstructed. From these cases, 17 arteries also included the side branch, which were finally selected for the current study: seven were left anterior descending arteries (LAD), six were right coronary arteries (RCA) and four were left circumflex arteries (LCX). From the LAD arteries, six side branches were classified as 9 and one as 1 according to the American Heart Association (AHA) classification. The side branches of the LCX arteries are classified as 12 according to the AHA. In the RCA arteries, the side branches are located between segments one and two. The average length was 65.8±31.7 mm and 22.8±4.6 mm of the main arteries and the side branches, respectively. All patients provided written informed consent before being recruited into the study.
The acquired CTCA data were processed using the QAngio® CT Research Edition, version 2.0.3 system (Medis Specials, Leiden, the Netherlands) and analysed offline by an experienced operator8 (Figure 2). The registration between the baseline and follow-up CTCA examinations was performed using anatomical landmarks such as the daughter branches. The plaque characterisation of necrotic, fibrous, fibro-fatty and calcified tissue was achieved using the validated methodology implemented in de Graaf et al10. In this methodology, the Hounsfield unit thresholds and the lumen attenuation values are utilised for the definition of the plaque components. The analysis includes only large daughter branches (lumen diameter >1.5 mm, length >20 mm) which originate from the epicardial coronary segments.
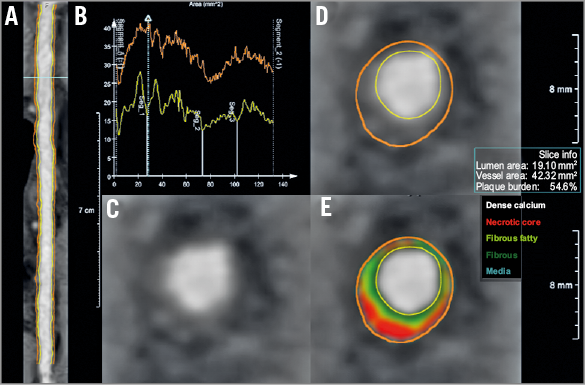
Figure 2. CTCA data process. A) Initially, the lumen and outer vessel wall borders were detected in the straightened multiplanar reformatted images for baseline and follow-up, respectively. B) Cross-sectional images are generated at 0.5 mm intervals. Gradient magnitude cross-section images (C) are then generated allowing accurate identification of the lumen and outer vessel wall border (D). An automated plaque characterisation method is used for quantification of four tissue types (E). Necrotic core is indicated with a red colour, fibrous fatty with a light green, fibrous with a green colour and calcific tissue with a white colour.
RECONSTRUCTION OF CORONARY ANATOMY
The detected lumen and outer wall borders were used for three-dimensional (3D) surface mesh representations. First, the straightened transformed images were converted back into their original orientations in order to calculate an implicit volume function. For each main and daughter branch an implicit volume function was extracted, where their merger was used to construct the coronary segment including the daughter branch. In a final step, the lumen and outer wall surfaces were generated and were treated using smoothing functions (Figure 3A, Figure 3B).
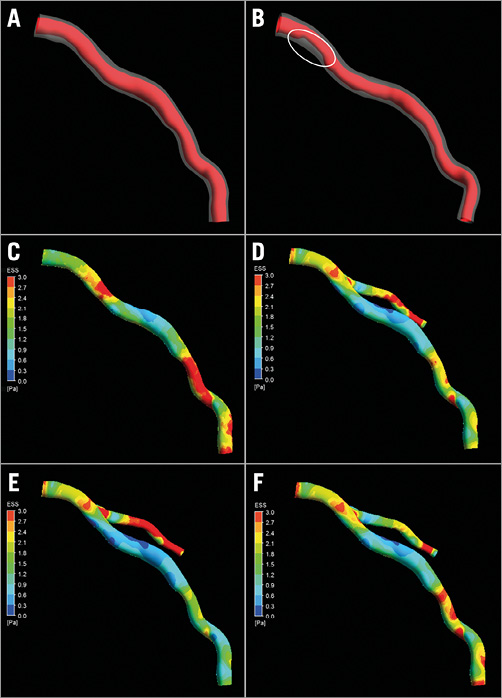
Figure 3. Example of arterial geometry for baseline (A) and follow-up (B). The white circle depicts the major progress of stenosis. C) - F) Endothelial stress for segment, Murray, Doriot and stress-free simulation case, respectively.
BLOOD FLOW AND BOUNDARY CONDITIONS
The Navier-Stokes equations were employed for blood flow modelling and estimation of the ESS (CFX 14.5; ANSYS, Inc., Canonsburg, PA, USA). The blood was assumed to be a homogeneous, Newtonian fluid with a dynamic viscosity of 0.0035 Pa·s and a density of 1,050 kg/m3. Blood flow was considered to be laminar and incompressible with a steady flow profile. Zero pressure conditions were applied at the inlet. Two angiographic runs acquired during PCI were used to define patient-specific coronary blood flow. The velocities were then used for the calculation of the flow rate using the area of the inlet boundary. The arterial wall was considered to be rigid and no-slip conditions were applied.
Three different flow division models were used to define a flow rate boundary condition at the outlet of the daughter branches. The first model assumed a stress-free profile with zero pressure boundary condition. The second model implemented Murray’s law to estimate flow in the side branches of the bifurcations assuming that the flow ratio through the side branches depends on the ratio of the diameter of the side branches to the third power11 given by:
![]() , (1)
, (1)
with qD1 and qD2 the flow through and dD1 and dD2 the diameters of the branches.
The third model was based on Doriot’s fit12 and takes into account the combination of the diameter and flow measurements in human coronary arteries:
![]() . (2)
. (2)
In order to implement Murray’s law and Doriot’s fit, the diameters of cross-sections of the branches after the bifurcation are estimated (Figure 4). Then, knowing the total inflow rate, the flow rates at the branches are calculated using equations 1 and 2. Finally, blood flow simulations were also performed in the main vessel omitting the side branch to examine whether the presence of the side branches affects the ESS and whether an incomplete coronary reconstruction would affect the accuracy of haemodynamics in predicting atherosclerotic evolution.
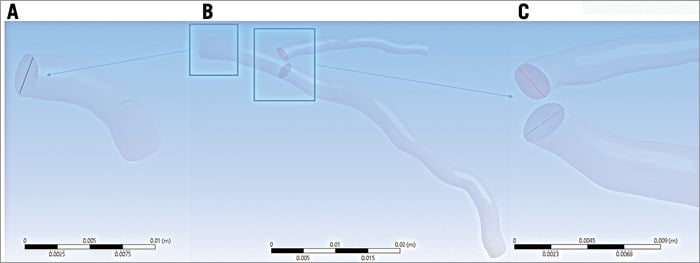
Figure 4. Use of Murray’s law. Initially the inlet diameter and area are calculated (A). Then the areas of cross-sections of the branches after the bifurcation are estimated (B) and, finally, the diameters of the branches are calculated (C).
DATA AND STATISTICAL ANALYSIS
Baseline and follow-up geometries were divided into 3 mm segments. This has traditionally been used to examine the association between ESS plaque characteristics and disease progression2. For each segment, the mean lumen area, the mean outer vessel wall area, the mean plaque area, the mean plaque burden (calculated as: 100x mean plaque area/mean outer vessel wall area), the mean composition of the plaque (necrotic core, fibro-fatty, fibrous and calcific tissue area) and the percentage of each plaque component as well as the local minimum averaged ESS in an arc of 90° were estimated2.
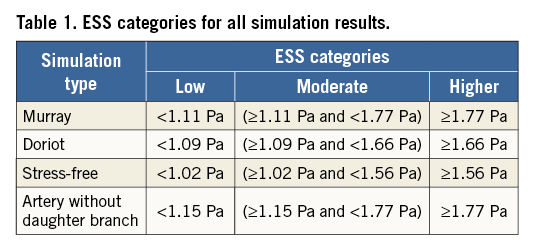
The predominant ESS values were divided into tertiles using the values of the entirety of all arteries to classify the 3 mm subsegments into those exposed to low, moderate and high ESS in each simulation scenario (Table 1). The baseline characteristics of these groups were compared using the Kruskal-Wallis H test for continuous variables and the chi-squared test for categorical variables. Linear and binary logistic regression analyses were performed to investigate associations between ESS and changes of lumen, plaque area and burden, and plaque characteristics at follow-up. We defined a significant change in lumen area as a decrease >14.5% from baseline (2 SD of the inter-observed variability of the expert who performed the analysis). Similarly, the 2 SD cut-off was used to define a significant plaque progression as an increase >14.7% of the plaque burden at follow-up. The values of the necrotic core change of the studied 3 mm segments were divided into quarters accordingly, and the upper quarter was used to define a substantial increase in the necrotic core. This was done because the 2 SD of necrotic core change is too low and could not be selected as an accurate cut-off threshold. To control for patient effect, a mixed model with random intercept and slope was used13. SAS version 9.3 (SAS Institute, Cary, NC, USA) software was used to perform the statistical analysis. A p-value <0.05 was considered statistically significant.
Results
Fifteen patients who had CTCA that enabled comprehensive assessment of coronary bifurcations (17 coronary arteries) at baseline and at three-year follow-up were included in the current analysis. The baseline characteristics of the studied patients are shown in Table 2. All the patients received dual antiplatelet treatment and routine standard of care therapy including statins at discharge. As is shown in Table 3, disease progression was noted at follow-up as the plaque area and the necrotic core component were increased at this time point. A typical example of blood flow simulations and ESS results is shown in Figure 3C-Figure 3F. There were small differences between the different simulation scenarios in the predominant ESS distribution in the 3 mm segments located proximally to the bifurcation segments. On the other hand, the differences of ESS distribution were higher in the main vessel distal to the bifurcation.
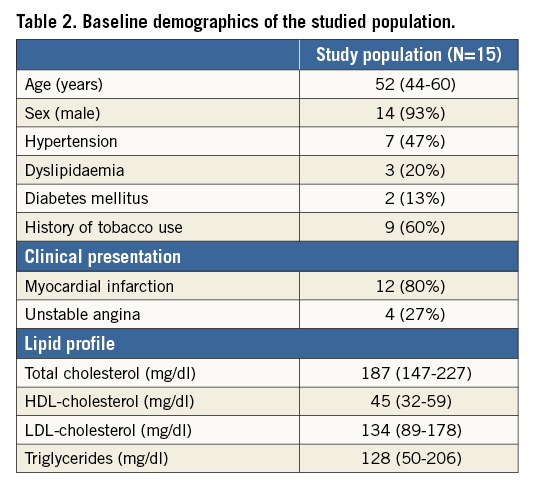
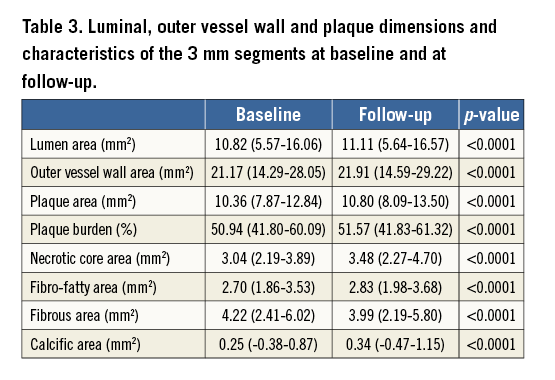
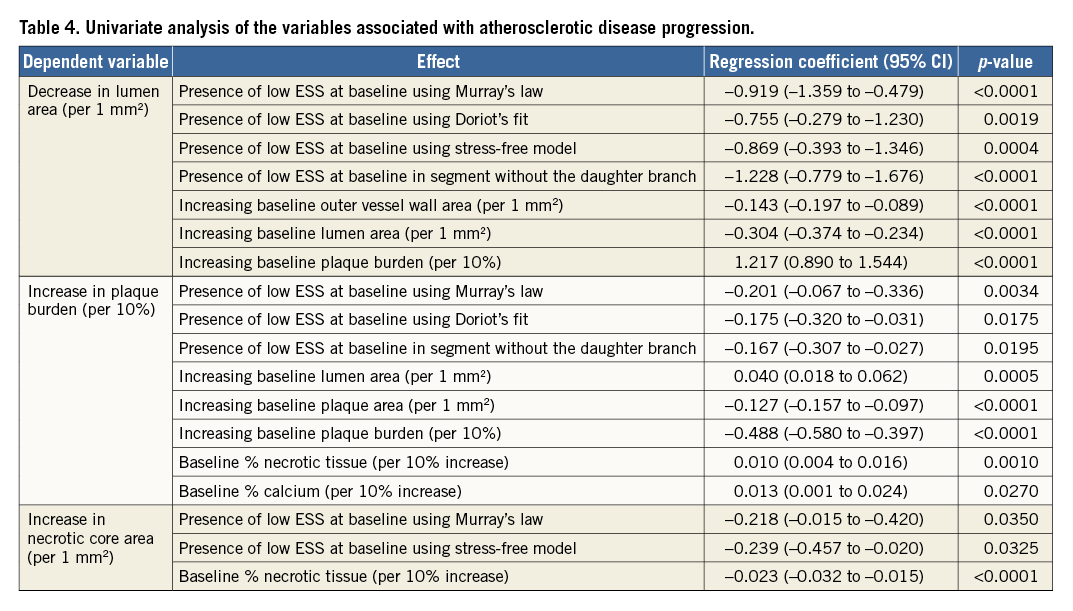
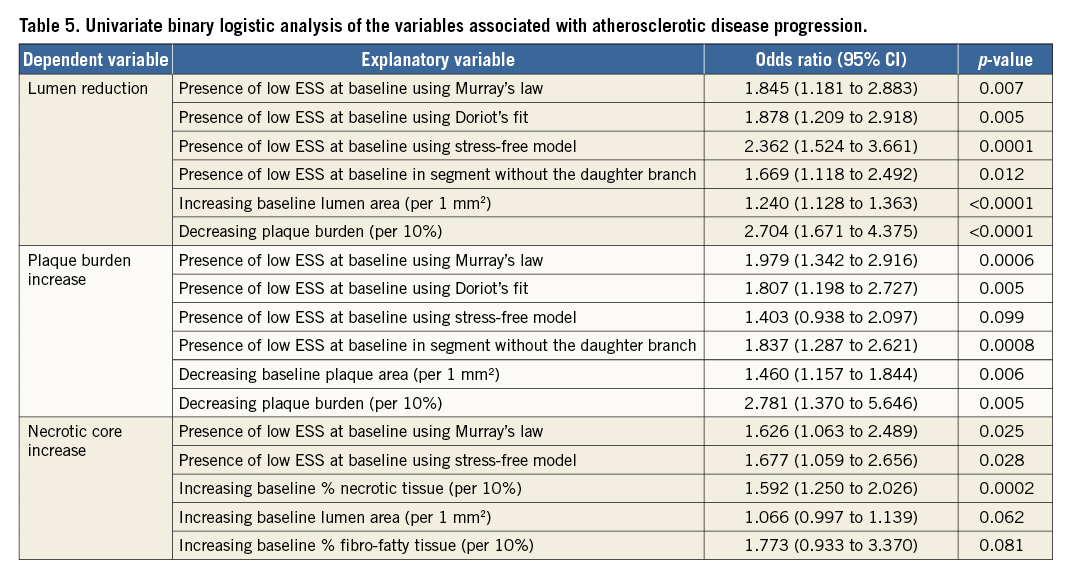
Table 4 shows the associations between the changes in lumen area, plaque burden and necrotic core component and the baseline ESS estimated in different simulations. Low ESS at baseline was associated with a reduction in lumen area irrespective of the flow division model. On the other hand, univariate linear regression analysis demonstrated an association between baseline ESS and plaque burden increase in the model without the daughter branch (p<0.0001), Murray’s model (p=0.0034) and Doriot’s model (p=0.0175), but not in the stress-free model. Necrotic core increase was correlated with baseline ESS in the Murray’s law model (p=0.035) and the stress-free model but not in the model without the side branch. Finally, the increase of fibro-fatty area was associated with baseline ESS in the Murray’s law model (p=0.0023), the Doriot’s model (p=0.0105), and in the model without the side branch (p=0.0197). None of the calculated ESS models was associated with the increase of the fibrous area.
The results of the univariate binary logistic analysis are shown in Table 5. Only the low ESS estimated in the Murray’s law model appeared as a predictor of significant lumen reduction (p=0.007), plaque burden increase (p=0.0006) and necrotic core increase (p=0.025). Low ESS in Doriot’s model was a predictor of lumen reduction and plaque burden increase but not of necrotic core increase, while low ESS estimated in the stress-free models predicted only the lumen reduction. Finally, low ESS in the model without the daughter branches was only a predictor of substantial lumen decrease and plaque burden increase at follow-up.
Discussion
In the present analysis, we investigated the effect of the side branches on ESS distribution in the main vessel and the effect of different flow division models in predicting disease progression in a retrospective data set of 17 coronary bifurcations from the PROSPECT-MSCT study. We found that blood flow simulation in models that included the side branches allowed more accurate prediction of atherosclerotic evolution and that the Murray’s law model simulation is the best for predicting changes in lumen dimensions, plaque burden and composition.
Several experimental and invasive imaging-based studies have examined the role of the local haemodynamic forces on atherosclerotic lesion formation and demonstrated that low ESS increases endothelial permeability and causes endothelial dysfunction, resulting in plaque growth2,3. Giannopoulos et al performed an ex vivo study in five hearts imaged using CTCA in order to demonstrate the effect of daughter branches on the distribution of ESS, which indeed changes in case of not including the daughter branch14.
Although ESS has numerous implications on vessel biology, the predictive value of low ESS especially for plaque progression has been proven only in some studies, while more commonly low ESS is associated with lumen reduction and plaque burden increase9. This could be at least partially attributed to the assumptions that are made during modelling. More specifically, the existence of the daughter branches affects the blood flow simulation considerably and thus the distribution of ESS5,14. Moreover, blood flow simulation is affected by approximations in the boundary conditions – which are a key factor for determining the local ESS.
Our study is the first to have performed complete coronary reconstruction from CTCA data including side branches to examine whether the ESS in these models can predict disease progression and investigate which model of flow division in coronary bifurcations provides the most accurate prediction of atherosclerotic evolution. In agreement with previous invasive imaging-based studies, we found that the ESS distribution estimated in models that included side branches was different from the ESS estimated in the models without the side branches15, and that the local haemodynamic patterns estimated in the complete reconstructions enabled more accurate prediction of atherosclerotic disease progression.
The definition of the boundary conditions is a key factor for reliable calculation of ESS16. In our study, we examined three different flow division scenarios and we found that the ESS estimated in models that included the side branches using Murray’s law enabled prediction of a decrease in the lumen area, and an increase in the plaque burden and necrotic core component.
Limitations
CTCA imaging was performed at baseline with an earlier-generation 64-slice scanner, which may have produced inferior image quality compared to the dual-probe scanner used at follow-up. Nevertheless, the tube voltage that may affect the performance of the plaque characterisation algorithm was the same in both scans. CTCA has limited accuracy in detecting plaque morphology and composition, while the blooming effect in calcified lesions may not allow accurate delineation of the lumen and outer vessel wall borders. However, in the present study the calcific tissue component was minimal and thus it is very unlikely to have affected the reported results. Another potential of CTCA is the ability to reconstruct the full coronary arterial tree. However, in this work we have included only diameters >1.5 mm and we focus only on the epicardial arteries. As a future work, the authors intend to implement blood flow modelling in coronary arterial trees in order to demonstrate the effect of ESS on disease progression under realistic geometric conditions.
A significant limitation of the present analysis is the small number of patients, which does not allow us to draw conclusions about the value of the ESS estimated in models including the side branches for predicting future adverse cardiovascular events. However, this is an exploratory study and, although we included only a small number of patients in the present analysis, the number of 3 mm segments (n=335) is sufficient to allow us to test the hypothesis of the current study. Moreover, the association of low ESS with plaque progression in a larger population of coronary arteries has already been proven in a previous work17. Another limitation of the current study regards the debatable argument about the CTCA capability to identify outer wall and plaque components. Indeed, CTCA has been validated in several studies concerning its accuracy to identify lumen, outer wall and calcified plaque components10,18,19. However, it has a limited accuracy to identify lipid tissue. On the other hand, the methodology for the plaque characterisation used in this study has been previously validated.
Conclusions
We performed blood flow modelling in 17 coronary bifurcations applying several kinds of boundary conditions to estimate flow division in coronary bifurcation. The implementation of flow division according to Murray’s law for the calculation of ESS allows more accurate prediction of atherosclerosis evolution (lumen area, increase in plaque burden and change in necrotic core) than using only the main vessels. Confirmation of these findings in future pilot studies is needed before conducting a large-scale study that would examine the additive value of the ESS estimated in models that include side branches in predicting lesions that will progress and cause events.
| Impact on daily practice This work proves that accurate computational modelling of blood flow in coronary arteries requires taking the daughter branch into account. Prediction of lumen reduction and plaque burden increase is associated with low ESS regions when the daughter branch is considered in the reconstruction, but also when omitting it from the reconstruction. However, the correlation of low ESS with necrotic core increase is present only when the daughter branch is considered in the simulation. |
Guest Editor
This paper was guest edited by Jolanda J. Wentzel, PhD; Department of Cardiology, Erasmus MC, Rotterdam, the Netherlands.
Conflict of interest statement
G. Stone is a consultant for Abbott Vascular, Volcano, and Infraredx. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

