Abstract
Aims: Hypertrophic obstructive cardiomyopathy (HOCM) with left ventricular outflow tract obstruction frequently involves a systolic anterior motion (SAM) of the anterior mitral leaflet. We hypothesised that SAM could be a new target for MitraClip therapy.
Methods and results: Three patients with HOCM were chosen for MitraClip therapy, due to significant SAM with subsequent mitral regurgitation. Invasive haemodynamic studies (right heart catheterisation, simultaneous transaortic pressure recording, and administration of nitroglycerine) were performed before and after MitraClip implantation, and a six-week follow-up was undertaken. MitraClip implantation was successfully performed with significant reduction of mitral regurgitation and SAM in all patients. Basal peak gradients (before clip: 65±25.5 mmHg; after clip: 7.7±5.0 mmHg) as well as provoked pressure gradients (before clip: 145.3±8.1 mmHg; after clip: 23.2±7.6 mmHg) were significantly reduced after MitraClip implantation. Right heart catheterisation data did not reveal major changes. At six-week follow-up, all patients presented in a persistently improved clinical state (NYHA Class I-II) with insignificant residual MR and continuously reduced LVOT gradients.
Conclusions: This is the first catheter-based study targeting primarily a SAM in HOCM to reduce LVOT obstruction. The results prove the concept that SAM is more than an epiphenomenon in HOCM. Thus, SAM-induced obstruction might be a valuable target for the MitraClip.
Introduction
There is an ongoing debate as to whether septal myectomy should be primarily performed using either surgical myectomy (Morrow procedure) or transcatheter instillation of alcohol into septal perforators for ablation of septal hypertrophy (TASH). In younger patients with hypertrophic obstructive cardiomyopathy (HOCM) the gold standard still seems to be surgical myectomy to relieve left ventricular outflow tract obstruction and cardiac symptoms1,2. Interestingly, HOCM is frequently accompanied by abnormalities of the mitral valve and the subvalvular mitral apparatus3. Usually, these abnormalities can be best managed surgically, without the need for mitral valve replacement. However, many surgeons still prefer mitral valve replacement (MVR), sometimes mechanical MVR, instead of mitral valve repair for patients with HOCM and concomitant degenerative mitral regurgitation (MR). In this regard, systolic anterior motion (SAM) of the anterior mitral leaflet usually causes MR, but may also be a significant contributor to LVOT obstruction. To prevent LVOT obstruction, extended septal myectomy is concomitantly performed with leaflet resection, leaflet plication and/or annuloplasty rings/bands4. In addition, an edge-to-edge stitch5 or transfer of posteriorly directed secondary chords (which would otherwise be resected) to the underside of the middle of the mid-anterior leaflet with a small piece of an anchoring pledget6 has been suggested.
Thus, the use of the edge-to-edge technology7, which has been transformed into a catheter-based procedure with the MitraClip device (Abbott Laboratories, Abbott Park, IL, USA), was especially appealing for us as a new treatment option for SAM in HOCM. Targeting a redundant anterior leaflet edge away from the LVOT by combining it with the posterior leaflet (A2 to P2) seemed to be very effective with the MitraClip. To prove this hypothesis, three patients with HOCM and significant SAM and dynamic LVOT obstruction were chosen for MitraClip implantation at our institution.
Methods
Between August 2012 and January 2013, three patients with HOCM and significant mitral regurgitation due to profound SAM were chosen to be treated with a MitraClip at our institution. Despite the relatively young age and low risk of these patients, a MitraClip approach as opposed to cardiac surgery was chosen as the first option within the Heart Team. The cardiac surgeons consented fully to the MitraClip approach for all patients, since repeated heart surgery (patient 1), anatomic criteria (patient 2) and concomitant disease (patient 3) were less favourable for surgery.
The first patient (male, age 69) had already been treated by surgical myectomy in addition to mitral ring annuloplasty (Carpentier-Edwards Physio II ring 30 mm; Edwards Lifesciences, Irvine, CA, USA) two years before. Despite a transient clinical improvement, he reported more recently having dyspnoea on slight physical exertion (NYHA Class II-III), and he complained about dizziness and palpitations most likely related to paroxysmal atrial fibrillation (PAF).
The second patient (female, age 78) had already been treated by a TASH procedure three years before, but she complained about several instances of syncope and dyspnoea at rest (NYHA Class IV) within the previous six months, which resulted in a panic disorder. Subsequently, she was treated by her primary care doctor with various doses of benzodiazepines without much benefit. Due to her physical discomfort she was largely inactive and became frail over time (BMI 16.2).
The third patient (male, age 76) had a classic past medical history of HOCM with multiple instances of syncope and dyspnoea on physical exertion (NYHA Class III). In the past he had several times refused to be treated by surgical myectomy and he had even refused an ICD implantation. More recently, he reported having angina at slight physical exertion (CCS class 3) and at his current admission to our department he presented with non-ST-elevation myocardial infarction (NSTEMI; troponin I 3,459 ng/L). A coronary angiogram was performed with subsequent PCI of a 90% left main stenosis. PCI was performed since the patient repeatedly refused to undergo surgical revascularisation.
All patients were investigated by routine transthoracic and transoesophageal echocardiography. Severe mitral regurgitation (MR) with significant SAM and LVOT obstruction was found in all cases (as assessed by continuous wave and pulse wave Doppler measurements). Invasive assessment (one pigtail placed in the ascending aorta, a second pigtail placed in the left ventricular apex) was used to assess the extent of dynamic subaortic obstruction. Post-extrasystolic pressure augmentation (pos. Brockenbrough-Braunwald-Morrow sign) and pressure responses after infusion of nitroglycerine (provoked pressure gradients) were measured across the LVOT before and after MitraClip implantation in all patients.
Coronary angiography disclosed significant residual coronary artery disease and several perforator branches were identified as possibly being suitable for a TASH procedure. A TASH procedure was discussed in all patients but judged to be a second choice (patient 1 had had a surgical myectomy, patient 2 had been treated by a TASH procedure, and patient 3 had recently been treated with a coronary stent extending across the first perforator branch). Thus, due to the profound SAM with a secondary MR, we were very interested in placing a MitraClip into the A2P2 segment of the mitral valve, in order to reduce not only MR but also the obstruction within the LVOT.
All patients were scheduled for a MitraClip (with TASH as a stand-by if edge-to-edge failed). The MitraClip procedure was performed as previously described7,8. Transseptal puncture was performed at 3.5 to 4.0 cm above the line of coaptation to make sure that the CDS would be able to capture a long anterior mitral leaflet deep inside the left ventricular cavity. With the use of 3D TEE, the clip was always oriented into the A2P2 segment, with precise perpendicularity to the line of coaptation. Grasping was attempted using of X-plane visualisation. Finally, 3D TEE was used to confirm if a broad tissue bridge was present between A2 and P2, and continuous wave Doppler measurements were used to rule out a significant mean transmitral gradient. Last but not least, the remaining resting LVOT gradient and the presence of a provoked pressure augmentation were tested in all patients after MitraClip implantation. At six-week follow-up, the patients underwent a control echocardiography for assessment of MR and measurements of the LVOT gradient.
Results
All three patients (mean age: 74.3±4.7 years, mean log EuroSCORE: 17.9±11.5) were referred with progressive shortness of breath (NYHA Class III). Transthoracic and transoesophageal echocardiography showed a significant SAM in patient 3 (Figure 1A, Figure 1B), and a variable extent of septal hypertrophy with diameters of between 19 and 24 mm in all patients (Table 1). Invasive assessment revealed a profound resting as well as dynamic subaortic obstruction in all patients (Figure 2, Table 2). After successful transseptal puncture, the clip delivery system (CDS) was oriented into the A2P2 segment using 3D TEE, with precise perpendicularity to the line of coaptation. Grasping of both leaflets was attempted in all patients with the use of X-plane visualisation. After a successful grasp, MR was almost completely abolished (before: 3.0±0; after: 0.5±0.5) in all patients. In addition, echocardiography demonstrated an impressive reduction of the systolic anterior motion (SAM) after MitraClip in patient 3 (Figure 3, Table 2). The clip was not deployed before an insignificant mean transmitral gradient was shown by continuous flow Doppler. Indeed, patient 3 (with an extremely long AML) demonstrated a significant mean transmitral gradient (7 mmHg) after initial clip closure, necessitating reopening of the clip. The clip was slightly relocated to the medial site of the A2P2 segment in addition to a more posteriorly directed guide position. All patients were treated by implantation of a single clip (final mean transmitral gradient: 3.6±1.1 mmHg) and the clips were deployed (device time 48.7±35.3 min) after confirmation of a broad tissue bridge between A2 and P2 (3D TEE). Most importantly, the MitraClip was able to blunt the gradients significantly in all patients. Basal peak as well as mean pressure gradients (±nitro) and post-extrasystolic (pos. Brockenbrough-Braunwald-Morrow sign) pressure gradients were significantly reduced after MitraClip implantation (Figure 2; example given of patient 3 in Figure 4A and Figure 4B). Right heart catheterisation data did not reveal major changes before and after MitraClip implantation. At six-week follow-up, all patients presented in a persistently improved clinical state (NYHA Class I-II), and echocardiography revealed insignificant residual MR with continuously reduced LVOT gradients.
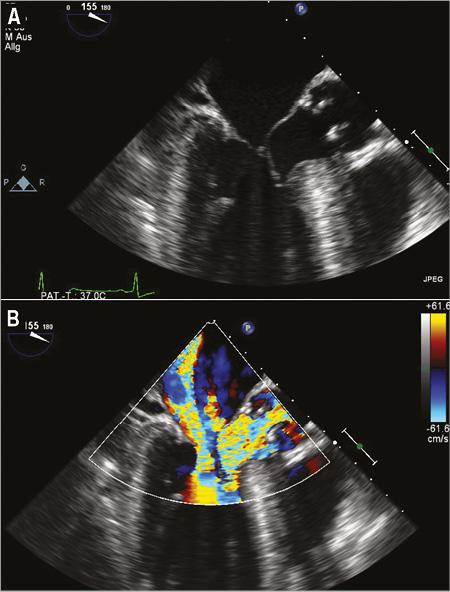
Figure 1. Patient 3. Transoesophageal echocardiography displaying significant systolic anterior motion (SAM) of the anterior mitral leaflet (A) in addition to significant mitral regurgitation with significant LVOT obstruction (B).
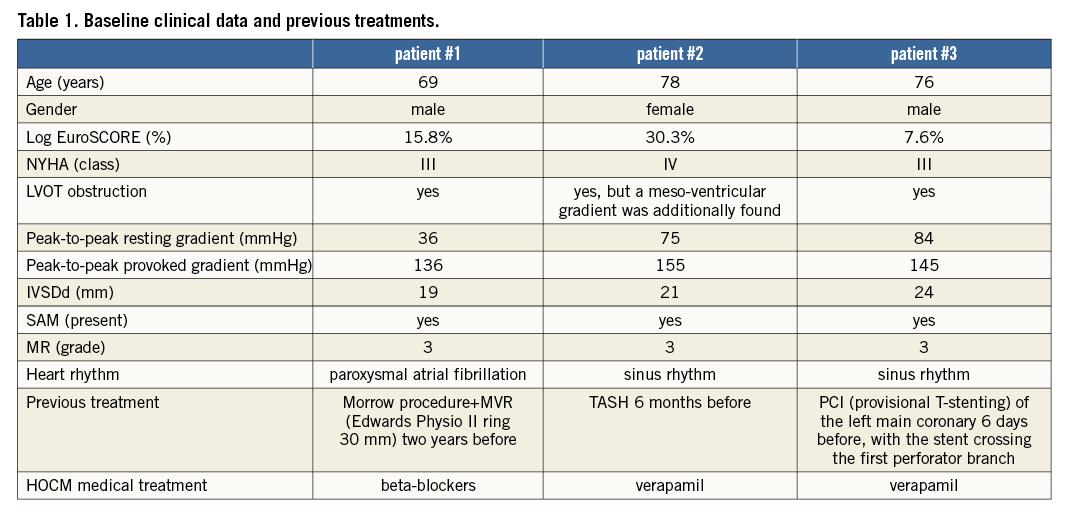
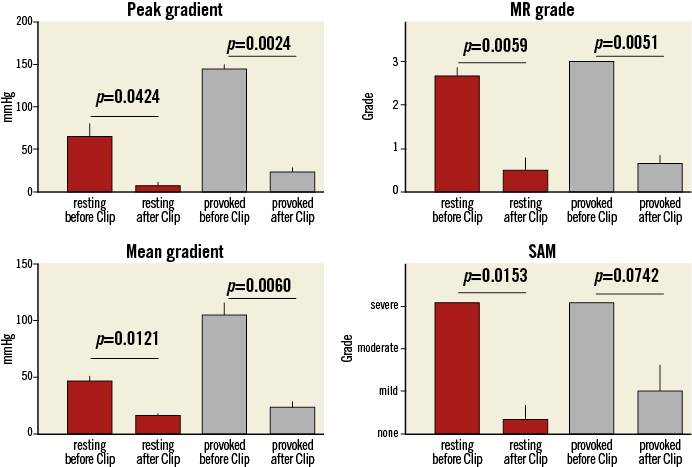
Figure 2. Invasive measurement of the average resting gradients (peak-to-peak and mean gradient), post-extrasystolic gradients (peak-to-peak and mean gradient) extent of mitral regurgitation and SAM before and after MitraClip implantation.
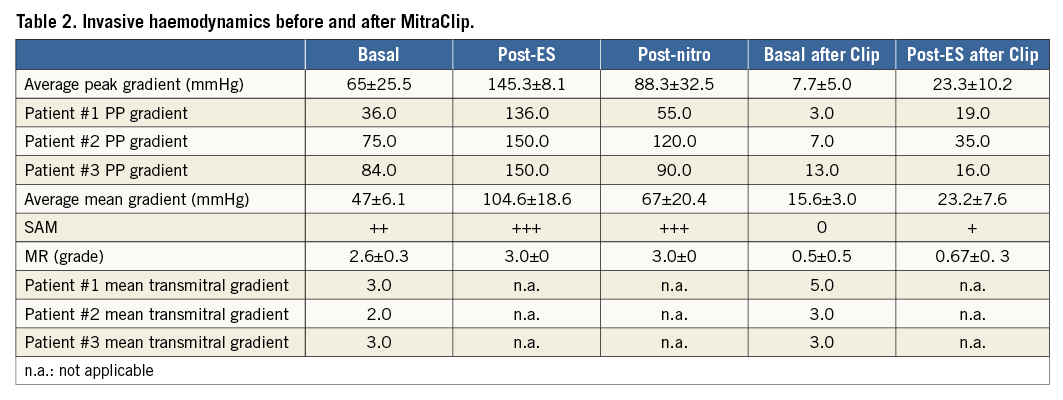
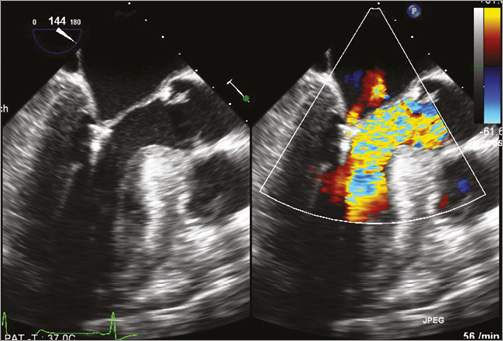
Figure 3. Patient 3. Transoesophageal echocardiography showing almost complete abolition of mitral regurgitation in conjunction with significant reduced LVOT obstruction. SAM of the anterior mitral leaflet is no longer visible.
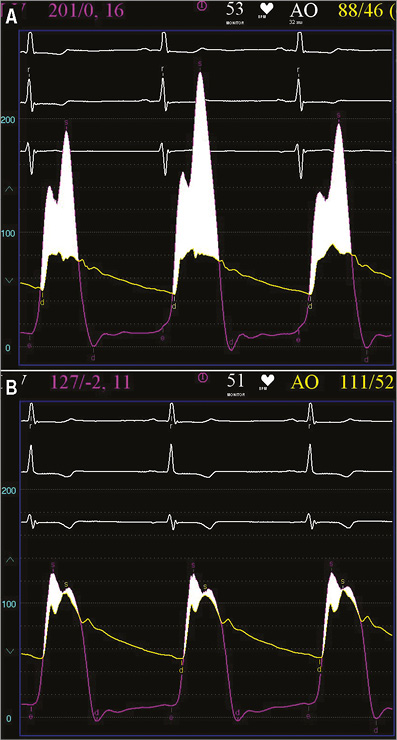
Figure 4. Patient 3. Invasive measurement of the basal transvalvular aortic gradient (aortic pressure vs. mid left ventricular pressure) before (A) and after MitraClip implantation (B), displaying a significant LVOT obstruction before treatment with a great reduction after mitral clipping.
Discussion
Over the last three years the MitraClip procedure has emerged as an efficient treatment option for mitral regurgitation in degenerative as well as functional mitral valve pathologies7-10. In addition, there is a strong trend for catheter-based technologies as alternative treatment options to open heart surgery in complicated pathologies or high-risk surgical candidates. To our knowledge, MitraClip implantation in patients with HOCM has so far not been described. Thus, this is the first report demonstrating that MitraClip implantation may also be considered in HOCM, if the presence of a SAM phenomenon can be attributed to LVOT obstruction.
Due to its lower invasiveness as opposed to surgical myectomy, the TASH procedure for HOCM is nowadays an accepted catheter-based technique. In this regard, a recent meta-analysis11 demonstrated a sustained decrease in resting and provoked LVOT gradient (65.3 minus 15.8 and 125.4 minus 31.5 mmHg, respectively) which was accompanied by a reduction of the basal septal diameter (20.9-13.9 mm), improvement in NYHA Class (2.9 minus 1.2), and an increase in exercise capacity (325.3 to 437.5 seconds). In reviewing the complications with TASH, early mortality (within 30 days) was 1.5% (0.0-5.0%) and late mortality (beyond 30 days) was 0.5% (0.0-9.3%). Other complications included ventricular fibrillation (2.2%), LAD dissection (1.8%), complete heart block requiring permanent pacemaker (10.5%), and pericardial effusion (0.6%). A second TASH procedure was necessary in 6.6% of patients, and 1.9% of the patients had to undergo surgical myectomy for resolution of symptoms. However, complete heart block seems to be the most frequent complication after TASH, reaching up to 20% in various reports12. Thus, albeit there is almost similar efficacy with regard to gradient reduction and clinical improvement with TASH compared to surgical myectomy, complete heart block occurs more frequently with TASH, favouring surgical treatment13 or possibly newer treatment strategies such as the MitraClip procedure.
After MitraClip implantation we found a much more pronounced gradient reduction for the resting gradient (65 minus 57 mmHg) as well as for the provoked gradient (145 minus 122 mmHg) in our study. In addition, we were clearly able to reduce mitral regurgitation and we demonstrated that LVOT obstruction was largely attributable to SAM-induced anatomical LVOT narrowing in these three treated patients.
Clearly, profound septal hypertrophy was not present in all patients. Thus, the MitraClip approach should be restricted to patients with significant SAM and a high likelihood of SAM-induced LVOT obstruction.
In general, coaptation of the MV leaflets depends on both anterior and posterior leaflet length and position, and the failure of either to adapt optimally in this setting usually results in mitral regurgitation or worsened LVOT obstruction. Thus, targeting SAM with catheter-based techniques is an appealing concept to treat LVOT obstruction and possibly also in other conditions in which SAM may contribute to gradient formation. Interestingly, SAM of the mitral valve causing LVOT obstruction occurs not only in HOCM. LVOT obstruction has also been observed in conditions such as hypertensive hypertrophy, dehydration, severe bleeding, sepsis, vasodilatation, excessive sympathetic stimulation, pericardial tamponade, and after aortic valve replacement for aortic stenosis, as well as after mitral valve repair. In general, acute SAM of the mitral valve can cause a life-threatening condition. SAM can result in critical LVOT obstruction and/or mitral regurgitation and is associated with a risk of sudden death (suicide ventricle) in up to 20% of cases. The mechanisms of SAM are complex and depend on the functional status of the ventricle. Most importantly, SAM has been described in 2%14 to 8%15 of patients undergoing mitral valve repair. In some cases, further surgery to relieve the obstruction is necessary. A likely contributing mechanism has been suggested to occur if mitral valve coaptation to septum distance had been surgically reduced by implantation of an over-downsized annuloplasty ring.
Besides the impressive observed relief on LVOT obstruction after MitraClip implantation, the transmitral gradient needs to be carefully monitored after closure of the MitraClip. As outlined above, HOCM is often accompanied by abnormalities of the mitral valve and the subvalvular mitral apparatus3,16. These HOCM-associated features (malpositioning of the papillary muscles, elongation and pathological thickening of the mitral valve) may significantly impact on the effective mitral orifice area after MitraClip implantation. In fact, we found some evidence that a pathological elongation of the anterior leaflet will result in a funnel-like structure deep below the mitral annulus. Relocation of a clip displaying an initially high transmitral gradient may resolve such an observation.
Conclusion
The edge-to-edge technique seems to be a very simple solution, since narrowing of the outflow tract due to dislocation of the coaptation zone towards the septum can be ruled out. Due to the lower invasiveness of the MitraClip as opposed to surgical MVR, further efficacy testing of a MitraClip approach in conditions with SAM might be of interest for the future.
| Impact on daily practice The present paper describes a new treatment option in selected patients and is presently considered as a new option in symptomatic HOCM patients at our institution. We systematically evaluate patients for eligibility (i.e., high surgical risk, profound systolic anterior motion and mitral regurgitation, not too profound septal hypertrophy). All treatment options are discussed with possible candidates and the off-label MitraClip approach requires a specific informed consent. |
Conflict of interest statement
U. Schäfer and K.H. Kuck are faculty members at the Cross Roads Institute, Abbott Vascular Laboratories in Brussels. The other authors have no conflicts of interest to declare.

