Abstract
Percutaneous alcohol septal ablation (ASA) is an effective and minimally invasive therapeutic strategy to resolve left ventricular outflow tract obstruction (LVOTO) in patients with hypertrophic cardiomyopathy who remain symptomatic on maximally tolerated medical therapy. First performed by Sigwart in 1994, the procedure consists in determining an iatrogenic infarction of the basal interventricular septum to reduce LVOTO and alleviate symptoms. Since its first description, numerous studies have demonstrated its efficacy and safety, proposing ASA as a valid and attractive alternative to surgical septal myectomy. The success rate of the intervention is profoundly affected by patient selection and centre experience. In this review, we sought to summarise current evidence on ASA, describing the procedure and proposing a cardiomyopathy team-based approach to resolve clinical disputes in clinical practice.
Introduction
Hypertrophic cardiomyopathy (HCM) is defined by an unexplained left ventricular hypertrophy, not solely explained by abnormal loading conditions1. Given the extreme heterogeneity, HCM has received over 70 different names by individual investigators since its original description. Between the 1950s and 1960s, a condition called asymmetrical septal hypertrophy with a high risk of sudden cardiac death (SCD) was described in the UK, while two gurus of modern cardiology, Braunwald and Morrow, first described and treated the dynamic left ventricular outflow tract obstruction (LVOTO) of a novel disease called idiopathic hypertrophic subaortic stenosis2. Detailed search methods used for this review are provided in Supplementary Appendix 1.
HCM is a common (prevalence 1:500), inherited disease in more than half of cases, with sarcomeric protein genes being the most frequent cause2. The diagnosis is based on echocardiography (or cardiac magnetic resonance) demonstrating the degree (left ventricular wall thickness >15 mm) and pattern (asymmetric, septal, concentric) of hypertrophy1. Differential diagnosis between HCM and athlete’s heart is challenging, and a multiparametric approach (including pre-participation ECG and imaging tests for competitive sports) is usually necessary to distinguish between physiological versus pathological hypertrophy3. A dynamic LVOTO is encountered in 30% of patients at rest, and another 30% after Valsalva manoeuvre, physical or pharmacological stress2. HCM patients can remain asymptomatic during most of their life course, unless they develop arrhythmias, left ventricular obstruction, diastolic or systolic dysfunction, consequently experiencing heart failure symptoms2. Even in patients with documented resting/provocable obstruction, the degree of symptoms can be extremely variable, making a clear classification of clinical status challenging. Life-threatening ventricular arrhythmias may be the tragic onset of the disease, particularly in young patients and apparently healthy athletes2,4. Thus, the SCD risk should be routinely evaluated according to clinical features and individualised risk calculators (i.e., HCM Risk-SCD model)4.
DIAGNOSTIC AND THERAPEUTIC WORKUP IN LVOTO
Left ventricular outflow tract (LVOT) gradient is dynamic and varies with loading conditions and contractility. The obstruction is caused by systolic anterior motion (SAM) of the mitral valve and mitral-septal contact2. Although initially considered pathognomonic of HCM, SAM is now recognised to characterise any conditions that alter mitral valve apparatus structure/function, mitro-aortic angle, and left ventricular structure/contractility (Supplementary Figure 1). In HCM, SAM has generally been explained by the “Venturi effect”, with a consequent decrease of the mitro-aortic angle5; however, primary abnormalities of the mitral apparatus (anterior-inward displacement of papillary muscles, leaflet elongation), and drag forces also play an important role. A unifying hypothesis suggests that, during ventricular systole, the Venturi effect may elevate the mitral valve, while drag forces facilitate an anterior displacement of the mitral valve. This synergistic mechanism pushes leaflets into the outflow tract, resulting in LVOTO and eccentric mitral regurgitation6. SAM is considered as severe if it accounts for more than 30% of systole.
LVOTO is defined by the presence of peak LVOT gradient >30 mmHg1. Echocardiography is the first-line technique in the diagnostic workup of hypertrophic obstructive cardiomyopathy (HOCM)1,2. M-mode echocardiography documents SAM, while continuous wave Doppler measures LVOT gradient showing a late-peaking systolic velocity (Supplementary Figure 1). Cardiac catheterisation may be required if there is discordance between symptoms and echocardiographic findings1, and peak-to-peak gradient at catheterisation most closely approximates instantaneous peak gradient by continuous wave Doppler echocardiography. European guidelines1 recommend investigating the presence of a latent obstruction in symptomatic HCM patients routinely (Supplementary Table 1). Although patients may generate large gradients under pharmacological provocation (dobutamine, nitrates), these manoeuvres do not reliably reflect the mechanism of obstruction; thus, exercise echocardiography represents the technique of choice for reproducing the presence of dynamic LVOTO7. The prognostic role of LVOTO remains debated as HCM-related death in patients with or without LVOTO is similar8. Accordingly, the obstruction should be regarded mainly as a determinant of clinical/haemodynamic status rather than a marker of ominous outcome1. Beta-blockers, non-dihydropyridine calcium channel antagonists, and disopyramide reduce LVOTO and alleviate symptoms by their negative inotropic/chronotropic effect. Dual-chamber pacing is also a feasible strategy for reducing LVOTO in selected cases with refractory symptoms and high-risk for surgical/interventional treatment9.
Alcohol septal ablation: historical, clinical and anatomic considerations
The concept of non-surgical interventional therapy for HOCM has evolved since the 1980s. The suggestion to use alcohol for inducing infarction of an hypertrophic septum is derived from the electrophysiology studies by Brugada on the treatment of ventricular arrhythmias using intracoronary alcohol injection10. Those studies inspired the Berlin cardiologist G. Berghöfer who, in 1989, first described the concept of an alcohol septal ablation (ASA) technique for HOCM (Berghoefer, personal communication). From the early 1990s, Gietzen and Kuhn described several cases of outflow gradient reduction after temporary balloon occlusion of the first septal branch10. In 1995, Sigwart first published three cases of percutaneous ASA in HOCM patients resistant to treatment, reporting the resolution of subaortic stenosis and improvement of symptoms from the day after the intervention10. To date, ASA is considered an effective, minimally invasive strategy in patients with LVOT gradient ≥50 mmHg and symptomatic HOCM despite maximally tolerated drug therapy1. It consists of an iatrogenic, localised infarction of the basal septum at the point of contact of the anterior mitral valve leaflet. Despite the absence of large-scale randomised studies, the growing volume of observational data has attracted clinical and interventional cardiologists, proposing ASA as an appealing alternative to surgical septal myectomy1.
CLINICAL CONSIDERATIONS
The first step in the decisional algorithm should include history collection and physical examination to assess clinical status and exclude other conditions (i.e., respiratory disease, thyroid dysfunction, anaemia) that might lead to misdiagnosis (Supplementary Figure 2). ASA is recommended in patients with moderate-to-severe symptoms (i.e., New York Heart Association Class III-IV, Canadian Cardiovascular Society grade III-IV angina pectoris), recurrent pre-syncope/syncope, or heart failure, which interfere substantially with lifestyle, despite optimal medical therapy1. The procedure might be of benefit in selected cases with mildly symptomatic and severe LVOTO, although further evidence in this setting is needed11. The preference for ASA according to clinical features (i.e., age, comorbidities, pacemaker presence, pre-existing right bundle branch block) depends on local expertise (Supplementary Table 2). ASA remains controversial in children and adolescents because of the lack of long-term data.
ANATOMIC CONSIDERATIONS: VENTRICULAR OUTFLOW AND CORONARY CIRCULATION
The evaluation of outflow geometry, septum morphology, and valve apparatus anatomy is crucial in predicting ASA feasibility, and the existence of LVOT, mitral valve and/or papillary muscle anomalies must be excluded. A septal thickness of ≥17 mm is the currently proposed cut-off by the European Guidelines to perform a safe procedure and minimise the risk of iatrogenic ventricular septal defect1. Of note, this recommendation is based on expert opinions. Definitive data in patients with modest hypertrophy (15-16 mm) are currently lacking; in these cases, an isolated mitral valve repair/replacement has been proposed as an alternative to septal reduction12. ASA efficacy may be inadequate in patients with severe hypertrophy (i.e., basal septum ≥25 mm) or extensive septal scar, due to the intrinsic limitation of alcohol10,13.
The coronary circulation anatomy and concomitant atherosclerosis should always be assessed preoperatively (and irrespective of angina) by invasive coronary angiography, to obtain information about the course and size of the coronary arteries and septal branches13. As an additional imaging technique, computed tomography coronary angiography can also provide further details with regard to coronary anatomy and septal vascular supply in preparation for ASA14.
The correct identification of a septal perforator branch with compatible anatomy for ASA is the cornerstone for a successful procedure. The first septal perforator artery is often chosen as target, perfusing in most cases the basal septum which is responsible for the greatest part of the obstruction13. It generally arises from the left anterior descending (LAD) artery and courses close to the His bundle and right bundle branch; non-LAD septal perforator branches are reported in 15% of cases and should be systematically screened15. If multiple culprit septal branches (identified by preprocedural or intraprocedural imaging tests) are present, they should all be ablated with multiple alcohol injections at index and/or staged procedures (if reintervention is needed). Inability to identify a satisfactory culprit septal branch occurs in approximately 10% of ASA candidates16. In some patients, no “optimal” septal artery for ablation can be identified due to the presence of multiple submillimetre septal branches not accessible to the necessary armamentarium15. In other cases, target septal branches also supply the free wall of the left ventricle, the papillary muscles or the right ventricle structures, similarly preventing the use of ASA.
Alcohol septal ablation: description of the procedure
ASA consists of selective infusion of 95-96° absolute alcohol into the septal perforator branch supplying the LV side of the basal or mid-cavitary septum13. The rationale is to determine an alcohol-induced occlusion of the vessel, with a controlled infarct in the basal septum that progressively turns from viable hypertrophic myocardium to thin akinetic scar, reducing LVOTO. Radial and femoral access are both feasible, and the choice mainly depends on operator preference and patient anatomy. The two approaches showed similar short- and long-term success rates, although the radial approach has been associated with lower rates of vascular complications17.
The main steps of the procedure are shown in Figure 1. After positioning an arterial sheath and temporary pacemaker, analgesic drugs (i.e., morphine) can be administered to control pain caused by alcohol injection and iatrogenic infarct.
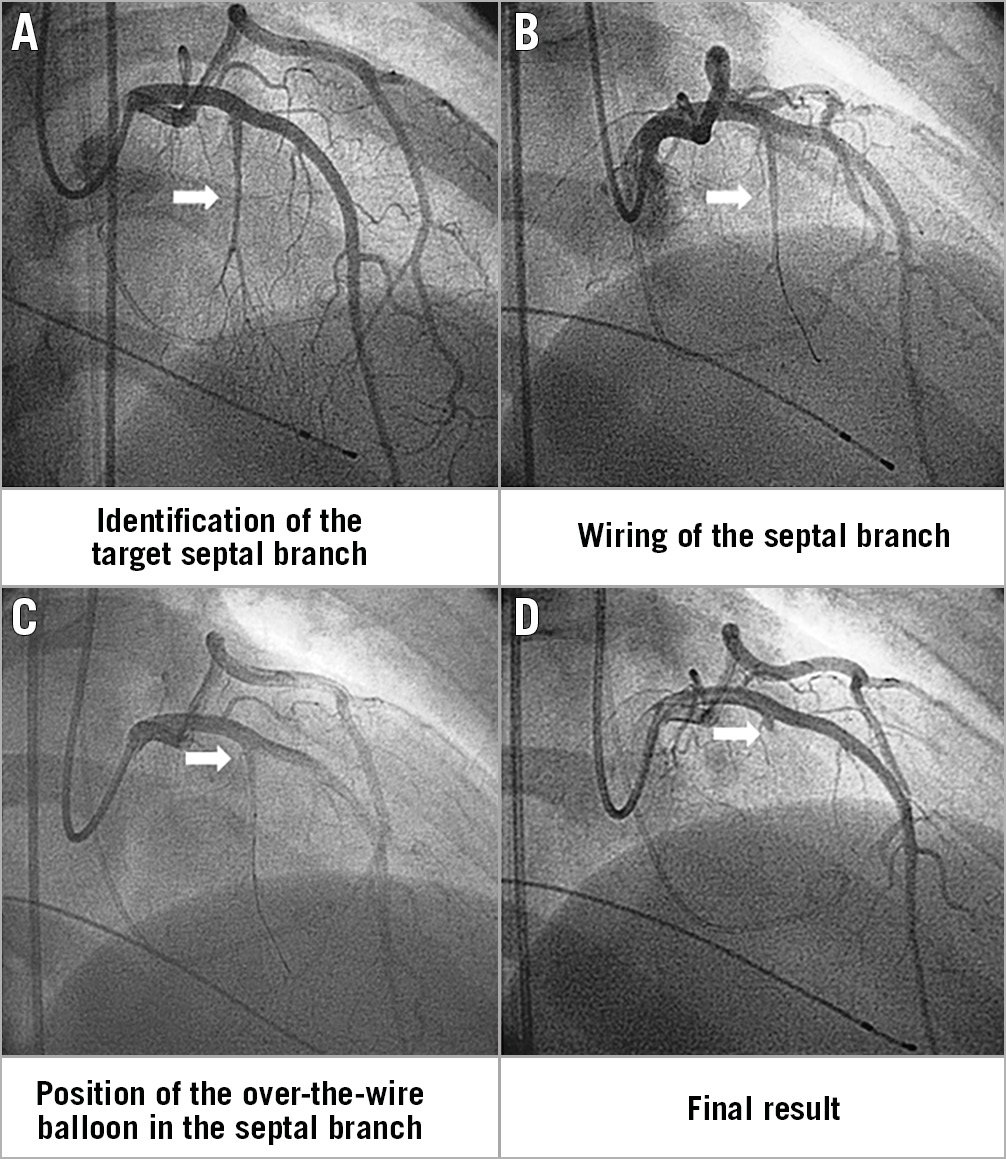
Figure 1. Description of the procedure. A) Left coronary angiography allows identification of the target septal branch (arrow). B) The septal branch (arrow) is wired. C) An over-the-wire balloon is positioned in the proximal part of the septal branch (arrow) for injection of echo contrast and alcohol. D) Coronary angiography at the end of the procedure demonstrates the septal artery stump (arrow) after alcohol-induced occlusion.
Diagnostic catheterisation may initially be performed to evaluate the LVOT gradient1,13. Coronary angiography is then performed to select the septal branch for ethanol infusion and assess vessel anatomy, origin, angulation, and size. Septal vessel course can be appropriately visualised through right anterior oblique or postero-anterior cranial projections, while the left anterior oblique view allows differentiating whether septal branches course along the right or left side of the septum (i.e., selection of left-sided branches reduces the risk of atrioventricular block)13.
After the engagement of the left main with a guide catheter providing extra support, a short over-the-wire (OTW) balloon (1.5-2.5 mm in diameter, 6-10 mm in length, with a balloon-artery ratio of approximately 1.3:1) is passed over a standard 180 cm 0.014-inch extra support wire and positioned into the target vessel. OTW balloons are recommended as they allow selective septal branch angiography during balloon inflation (1-2 ml of contrast slowly injected in the proximally occluded vessel) to test the correct positioning, complete septal occlusion, and absence of contrast reflux into the LAD. Due to high collateralisation between the left and right coronary, excluding the filling of any other coronary arteries through septal collaterals before alcohol injection is mandatory18. Then, the target vessel must be tested with myocardial contrast echocardiography (Figure 2): 1-2 ml of echocardiographic contrast must be injected through the OTW balloon to visualise the target area and exclude contrast misplacement in other regions (i.e., inferior wall, papillary musculature, right ventricle), which represents an absolute contraindication to ethanol infusion19. Among echocardiographic contrast agents, first-generation Levovist® has been widely used, but is no longer available in many countries. Second-generation agents are rapidly washed out without formation of a good depot in the myocardium; Gelafundin®, a volume expander with good echocardiographic contrast, is also suitable for ASA. In more challenging cases, intracardiac echo or three-dimensional (3D) contrast echocardiography can be useful for intraprocedural guidance20.
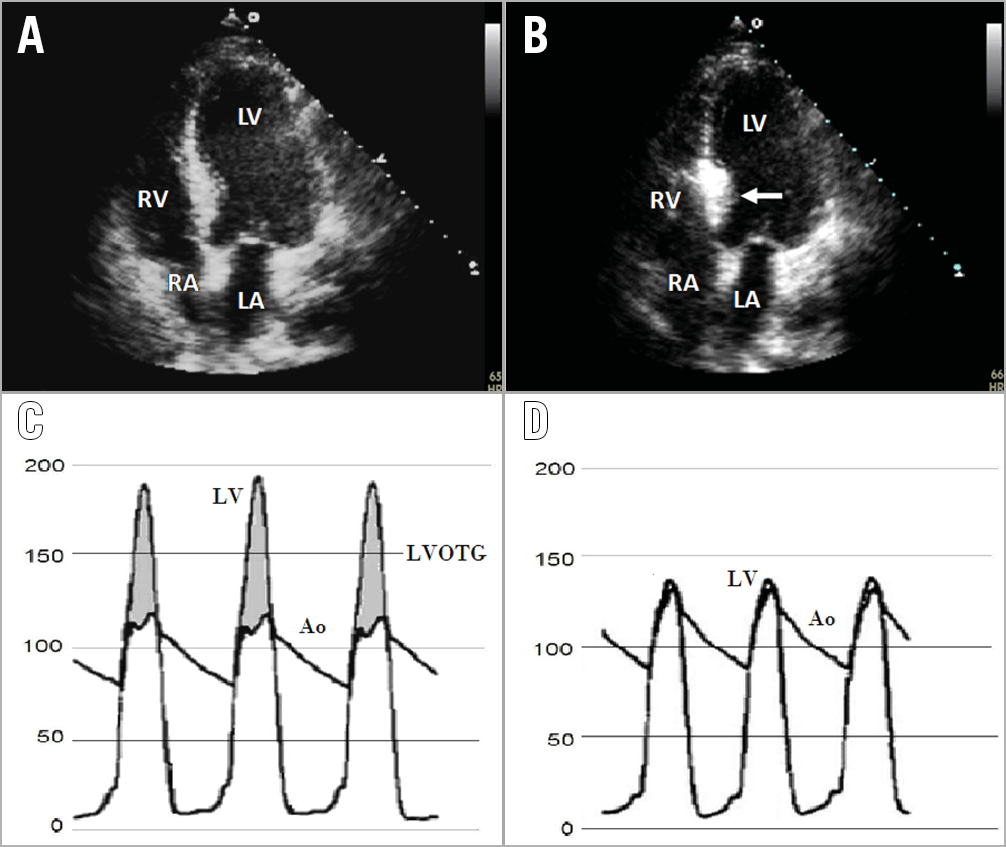
Figure 2. Periprocedural monitoring. A) Transthoracic echocardiography before ASA showing asymmetric septal hypertrophy. B) Localisation of echo contrast (thick arrow) in the basal septum in apical four-chamber view. Cardiac catheterisation pressures showing the LVOTG between the LV and aorta before (C) and after ASA (D). Ao: aorta; LA: left atrium; LV: left ventricle; LVOTG: left ventricular outflow tract gradient; RA: right atrium; RV: right ventricle
Thereafter, the operator can proceed with 1-3 ml ethanol injection over a one- to five-minute period in the target vessel1. The amount of alcohol is about 0.7-1 ml for every 10 mm of measured septal thickness21. During ethanol infusion, the inflated balloon must be firmly placed to occlude the vessel completely and avoid extensive myocardial damage20. Aggressive injection is discouraged as ethanol may traverse through collaterals and create inferior wall injury. Final coronary angiography excludes coronary injuries.
PROCEDURE EFFICACY: DEFINING A SUCCESSFUL ABLATION
The aim of ASA is to remove LVOTO and obtain a significant and sustained reduction in resting/provocable gradients (≥50% of baseline at six-month follow-up) (Supplementary Figure 3) and symptoms. Multinational studies have shown that after ASA approximately 90% of patients are in New York Heart Association Class I-II, the mean decrease of LVOTO is 76%22,23, and need for reintervention about 7%24. Benefits may take up to 12 months to become clinically evident, particularly in younger patients25. Although a correlation between alcohol dose and the area of myocardial necrosis measured by cardiac biomarkers has also been reported (peak creatine kinase of 500-1,000 U/L per millilitre of ethanol injected)26, cardiac enzyme levels have not been proved to predict procedural success or LVOT gradient reduction at follow-up26. The potential prognostic impact of ASA for reducing the risk of cardiovascular events is still a matter of debate24,27, although residual LVOTO has been independently associated with an increased risk of arrhythmias and mortality27.
PROCEDURAL SAFETY AND COMPLICATIONS
The most frequent complication related to ASA is transient or permanent complete atrioventricular block (approximately 30% and 10% of cases, respectively)22,24, due to the anatomical proximity of septal perforators with the conduction system. Complete atrioventricular block may also develop later (either as a delayed occurrence or a recurrence after recovery), especially in patients with advanced age or prolonged QRS. Thus, in selected cases, a temporary prophylactic pacemaker should be prolonged up to six days28. Pacemaker implantation is indicated if conduction disturbance persists for >24-72 hrs13. Since right bundle branch block occurs in more than 50% of cases, preprocedural pacemaker implantation might be considered in patients with pre-existing left bundle branch block27. In candidates for ASA, with a concomitant indication to receive an implantable cardiac defibrillator, device implantation should precede ASA in order to simplify post-procedural management. Of note, the HCM Risk-SCD model has been validated in patients undergoing ASA29.
Other potential complications are the infarction of the anterior wall, papillary muscles, or right ventricle due to collateral septal flow to the right coronary artery or LAD30. As coronary occlusion during ASA may lead to immediate recruitment of collateral circulation, a single bolus injection of ethanol is suggested. If a second injection is needed, the presence of collaterals should be carefully re-checked before the additional injection30. The occurrence of procedure-related mortality is less than 1% (similar to surgical myectomy)22,23. Concerns have been raised about the association between higher alcohol dose (>2 ml) and worse prognosis26. Potential explanations could lie in the more extended infarct scar due to higher alcohol dose that can predispose to a higher risk of atrioventricular block and life-threatening arrhythmia. However, although some authors have reported a fivefold increase in the risk of ventricular arrhythmias with ASA compared with septal myectomy31, this issue remains controversial. Proper patient selection and the use of low alcohol dose remain central to reduce the risk of complications.
Septal ablation for HOCM: personalised treatment
The choice of therapy should always be made on an individual basis with a multifactorial approach10. Shared decision making with patients should always be pursued, discussing the risks and benefits of each approach, then understanding the needs and preferences of the individual patient. In order to choose the best personalised treatment, it is crucial that the decision process is carried out with a multidisciplinary approach by a cardiomyopathy team working in dedicated cardiomyopathy centres of excellence.
CARDIOMYOPATHY TEAM
The concept of the “Heart Team” has been shown to improve decision making in coronary artery and valvular heart disease. Similarly, for HOCM, an experienced multidisciplinary “Cardiomyopathy Team” should analyse diagnostic evidence, put into context the clinical condition of patients, determine the need for interventions and the likelihood of safe and effective septal reduction with either ASA or myectomy. This team should ideally be composed of at least one clinical cardiologist, an interventional cardiologist, and a cardiac surgeon with recognised experience in the management of HOCM.
CARDIOMYOPATHY CENTRE
Results of both ASA and myectomy are largely dependent on the experience of the institutions to which patients are referred. When performed by experienced operators, ASA has been demonstrated to be safe and effective1, although long-term data remain limited compared with surgical myectomy24. As regards myectomy, recent data suggest that the real-world mortality rate associated with myectomy is approximately 4-16%, as compared with <1% found in the best high-volume centres23. As regards ASA, a recent multicentre study has found that an institutional experience of >50 ASA procedures was associated with lower occurrence of complications, better cardiovascular survival, better haemodynamic and clinical effect, and less need for repeat interventions32. The American guidelines for HCM (Supplementary Table 2) recommend that septal reduction therapy - either septal myectomy or ASA - should be performed only by experienced operators in the context of a comprehensive HCM clinical programme, with the goal of a <1% operative risk for isolated septal myectomy and a major complication rate <3%33. To date, HCM centres with high-volume surgical programmes performing myectomy are not universally available. Moreover, procedural volumes are still low in most hospitals, and deviations from guidelines may result in critical issues23. Although specific data are lacking, a minimum of 10 ASA or 10 septal myectomies per operator per year seems to be a reasonable caseload to be required to maintain competence in septal ablation therapies1.
Current limitations and future perspectives in LVOTO treatment
Although ASA represents a consolidated strategy, several limitations persist. The procedure-specific complication rate (particularly atrioventricular block) remains relevant. Long-term data supporting ASA are limited compared with surgical myectomy. Ultimately, operators with adequate experience are not widely available. To improve current practice in septal reduction, novel approaches have also been suggested. Percutaneous intramyocardial septal radiofrequency ablation is a safe and effective alternative to treat LVOTO, with a very low risk for conduction system injury34; however, further studies are needed to compare novel and standard techniques. More data are also required to clarify whether routine preprocedural computed tomography coronary angiography and intraprocedural guidance with additional imaging techniques (i.e., 3D or intracardiac echocardiography)20 might improve practice in patients with challenging anatomy.
Conclusions
Two decades on from its introduction, ASA has proven to be effective and safe in patients with HOCM. Studies comparing ASA and myectomy have reported similarly low rates of complications, especially when performed in centres of excellence. As a weak point, the rates of reintervention and pacemaker implantation remain higher for ASA. In the near future, a multidisciplinary “Heart Team” approach, based on the consensus of clinicians, interventionalists, and surgeons with recognised expertise in HOCM, has the potential to improve decisional strategy. Finally, the establishment of “cardiomyopathy centres” with high volume and dedicated skills should be considered to reduce procedural complications and improve outcomes in this special population. Further research is needed to assess the impact on daily clinical practice of these implementation strategies.
Conflict of interest statement
The authors have no conflicts of interest to declare.

