Background
Hypertrophic cardiomyopathy (HCM) is a relatively common disorder with a prevalence of approximately one in five hundred. It is associated with an increased risk of arrhythmic sudden cardiac death and heart failure either from diastolic (or in later stages systolic) dysfunction or left ventricular outflow tract obstruction with or without mitral regurgitation. At rest a left ventricular outflow tract obstruction is seen in 25% of patients. Mainstays of medical management are beta-blockers, calcium channel blockers and disopyramide. Alternatively right ventricular pacing can be considered. The reduction in outflow gradients achieved with medical therapy and pacing is modest at best and, therefore, some patients continue to experience important lifestyle limiting exertional dyspnoea. Moreover, the presence of an outflow tract gradient at rest has been associated with a significant increase in cardiovascular mortality. This is the basis for percutaneous alcohol septal ablation. It has been demonstrated to significantly reduce or eliminate left ventricular outflow tract gradients as well as related symptoms.
Indications
Appropriate patient selection is critical for success and avoidance of adverse events. Recommendations vary somewhat regarding the minimal gradient and functional class required. Currently, most would agree that myectomy or septal ethanol ablation is reasonable in symptomatic patients (class III-IV NYHA or CCS dyspnoea or angina, or recurrent exercise-induced syncope) in the presence of a septal thickness of at least 18 mm and peak instantaneous gradient at rest or with exertion of at least 50 mmHg. Others have recommended at least a 30 mmHg gradient at rest or 60 mmHg with exercise.
Methods
Coronary angiography is performed either via femoral or radial access (Figure 1) and a temporary pacemaker wire positioned in the right ventricle. Via separate arterial access, a pigtail catheter is positioned in the left ventricular apex to allow simultaneous left ventricular and aortic pressure measurements. The left coronary artery is engaged and a baseline outflow tract gradient is recorded invasively and echocardiographically. Anticoagulation with IV heparin is administered. Adequate sedation and opioid analgaesia is recommended due to expected pain related to septal ischaemia and injury. A coronary moderate support guidewire is then positioned into the first septal perforator. Subsequently, a compliant over-the-wire balloon is advanced over the coronary guidewire into the septal perforator. It is inflated to nominal pressure and coronary angiography is performed to demonstrate septal perforator occlusion and confirm uncompromised LAD flow (Figure 2). The coronary guidewire is subsequently removed and contrast (1-2 ml) injected vigorously through the central lumen of the balloon catheter to opacify the septal perforator and to assure absence of contrast reflux proximal to the balloon. If the location of predicted injury is satisfactory 96% ethanol is injected at no faster than 0.1 ml per minute for a total of approximately 1-2 ml. Final angiography is performed (Figure 3).
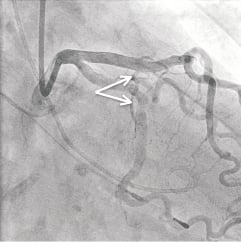
Figure 1. Angiography demonstrating the first septal perforator (two arrows). A pigtail catheter is positioned in the left ventricle and a temporary pacemaker wire in the right ventricular apex.
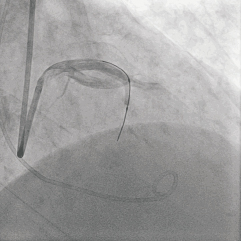
Figure 2. A coronary balloon is inflated in the septal perforator and angiography performed to assure uncompromised flow in the left anterior descending coronary artery.
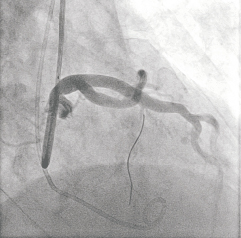
Figure 3. Final angiography demonstrating an occluded septal perforator and uncompromised flow in the left anterior descending coronary artery.
Difficulties
Anticipated difficulties are conduction disturbances, inadvertent alcohol injection or reflux into the parent vessel, coronary perforation, ventricular septal defect and ventricular arrhythmias.
More detailed information regarding background, indications, methods and difficulties is available in the online article.
Conflict of interest statement
The authors have no conflicts of interest to declare.

