
Abstract
Aims: Alcohol septal ablation (ASA) is an established treatment option in hypertrophic obstructive cardiomyopathy (HOCM). ASA is ineffective in some: inaccurate infarct and inability to identify a vessel contribute. We aimed to improve accuracy of infarct using CT angiography guidance and provide a more predictable and satisfactory outcome.
Methods and results: Twenty-one successive patients with symptomatic LVOT obstruction refractory to medication underwent CT angiography planning to guide ASA. CT was performed using a dual-source CT system. Alcohol was delivered to the artery identified from CT: in 17/21 this was a sub-branch of a septal artery, in 2/21 the septal vessel was identified from the circumflex artery. Peak gradient improved from 98 (IQR 89.50-111.50) mmHg to 14 (IQR 8.50-22) mmHg (p=0.003). Systolic anterior motion (SAM) improved in 18/20 patients. NYHA class improved by ≥1 in 18/20. Peak VO2 improved from 79.19% of predicted value (±14.01) to 91.62% (±12.02) predicted (p<0.0001). Success at the first procedure is greater with CT guidance, 17/20 vs. 50/75 with traditional methods (pre-CT guidance) (p=0.02); 9/20 had six-month CMR with target septum infarct in all. ASA-related RBBB reduced from 62% to 13% (p=0.0004).
Conclusions: CT angiography planning improves localisation of infarct and procedural success at the first attempt in ASA when compared to traditional methods. Follow-up to six months suggests a symptomatic, functional and haemodynamic improvement.
Abbreviations
ASA: alcohol septal ablation
CT: computed tomography
HOCM: hypertrophic obstructive cardiomyopathy
LAD: left anterior descending
LV: left ventricle
MV: mitral valve
NYHA: New York Heart Association
RCA: right coronary artery
RV: right ventricle
SAM: systolic anterior motion
Introduction
Hypertrophic cardiomyopathy (HCM) is an inherited disease characterised by otherwise unexplained hypertrophy of the myocardium. Whilst the distribution of hypertrophy can be variable, involvement of the basal interventricular septum is common. This anatomical pattern is associated with the development of left ventricular outflow tract (LVOT) obstruction, present in 20-30% of subjects at rest and in 70% on exercise or with provocation1. Systolic anterior motion (SAM) of the mitral valve is critical to the pathophysiology of LVOT gradient development in hypertrophic obstructive cardiomyopathy (HOCM). SAM-septal contact leads to an amplifying positive-feedback loop which further narrows the LVOT. This results in increasing LVOT flow velocities and gradients2. LVOT obstruction is associated with greater levels of dyspnoea, a greater incidence of stroke and higher mortality1. Treatment with alcohol septal ablation (ASA) can improve symptoms and has been suggested to improve prognosis3.
ASA causes death of myocardial tissue akin to a myocardial infarction. The reduction in size and systolic excursion of the septum leads to an increased LVOT area. The target for the iatrogenic infarct is the site of contact of the anterior mitral valve leaflet on the septum (SAM-septal contact). This reduces SAM and therefore gradients. Failure to resolve the LVOT gradient is seen in a substantial proportion of patients4,5. This can occur with inaccurate infarct location. Better localisation of iatrogenic infarct could lead to a more predictable and satisfactory outcome from ASA.
Current procedural methods rely on identifying a target vessel from invasive angiography and investigating with myocardial contrast echocardiography6. Invasive angiography provides information about the course and size of coronary arteries, but cannot provide information about the territories supplied. Computed tomography (CT) angiography has the dual benefit of detailing vascular anatomy and providing information on myocardial distribution. CT has been used in an exploratory manner to assist in ASA previously7. We describe a new method using CT angiography to describe septal vascular supply in preparation for ASA. This highlights the specific optimum target vessel (usually a branch division of the septal system) for alcohol delivery. We also describe outcomes in a consecutive series of patients undergoing ASA guided by CT, and compare this group to historic controls.
Methods
PATIENT SELECTION
Twenty-one consecutive patients who received alcohol to the target septal artery are described. A patient consort diagram is provided in Figure 1. A diagnosis of HCM was made according to typical clinical, electrocardiographic and echocardiographic features. All patients taken to the laboratory had resting, Valsalva manoeuvre or exercise stress peak LVOT gradient ≥50 mmHg and basal interventricular septal diameter ≥15 mm. All were trialled on medications prior to ASA. The mean age was 57.41 (±14.84) years, and 62% were male. One patient had significant lung disease, two had previous PCI. Three patients had undergone ASA previously with unsatisfactory outcome.
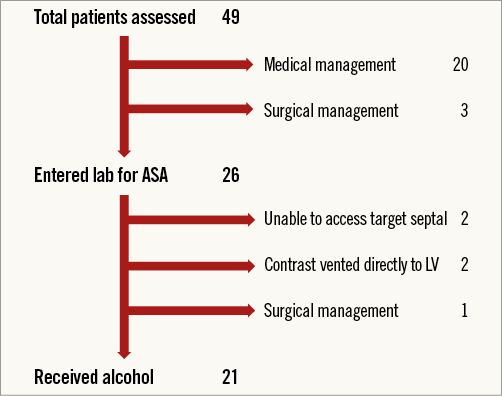
Figure 1. Patient consort diagram: all patients referred for ASA.
Appropriate permissions to perform CT prior to clinically indicated ASA were provided by the research and development board of Liverpool Heart and Chest Hospital.
ECHOCARDIOGRAPHIC ASSESSMENT
A resting echocardiogram was performed >24 hours prior to ASA (Philips iE33 scanner, Philips S5-1 probe; Philips Healthcare, Best, the Netherlands). SAM severity grading was adapted to provide a binary option of “contact with septum” or “no contact with septum” to allow statistical analysis. This is based on the observation that the majority of the increased flow velocity in the LVOT is related to SAM-septal contact2.
CARDIOPULMONARY EXERCISE (CPEX) TESTING
CPEX was performed using a bicycle ergometer with 10 W minutely increments in workload. Patients were exercised until a respiratory exchange ratio (RER) of >1.1 was reached; mean readings of the last 30 seconds of exercise were used.
CT IMAGE ACQUISITION
Coronary computed tomography angiography (CTA) was performed using a dual-source CT system (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany). All patients with a heart rate greater than 60 beats per minute were given either oral or intravenous metoprolol. Axial data acquisition was prospectively triggered at 70% of the RR interval. A lower 25% dose was given during 30 to 80% of the cardiac cycle to obtain systolic frames and SAM-septal contact area.
To synchronise acquisition of the data set to arterial enhancement, a test bolus protocol was used; 15 mL contrast agent (Optiray™ 350; Covidien/Medtronic, Dublin, Ireland) was followed by 40 mL saline solution at 6 mL/s. The time to peak enhancement in the aorta was measured using a series of transaxial scans acquired in 2 s increments, with the first image acquired after 12 s. For CTA, 60 mL of contrast was injected followed by a mixed flush (10 mL contrast and 30 mL saline), both at flow rates of 6 mL/s. For image reconstruction, a half scan reconstruction algorithm was used, providing a temporal resolution of 75 ms. The reconstructed slice thickness was 0.6 mm, and the slice increment was 0.3 mm.
CT IMAGE ANALYSIS
The reconstructed images were analysed with syngo.via (Siemens Healthcare). The target area of myocardium was identified using a short section of systolic imaging (Figure 2). This target myocardium at the SAM-septal contact area was examined in diastole to identify a segment of its arterial branch supply (Figure 2, Figure 3). This vessel was tracked back to its parent artery. Other characteristics of the septal vessel including the angle of bifurcation from its parent artery, the length of its course in epicardial fat (before septal penetration) and myocardium, its branch pattern, and the ultimate myocardial territories of all branches were noted (Figure 3). The target artery was traced and marked and a coronary angiogram “map” created. The coronary map was then rotated through horizontal and vertical planes to allow optimal visualisation and remove any overlap or foreshortening (Moving image 1). The optimum angiographic projections were then used as the “working views” in the catheterisation laboratory (Figure 4). Finally, the left main stem, LAD, circumflex and RCA were surveyed for other vessels tracking towards the septum. All potential target vessels were followed and the ultimate distribution noted. This was to allow identification or rejection as an additional target vessel.
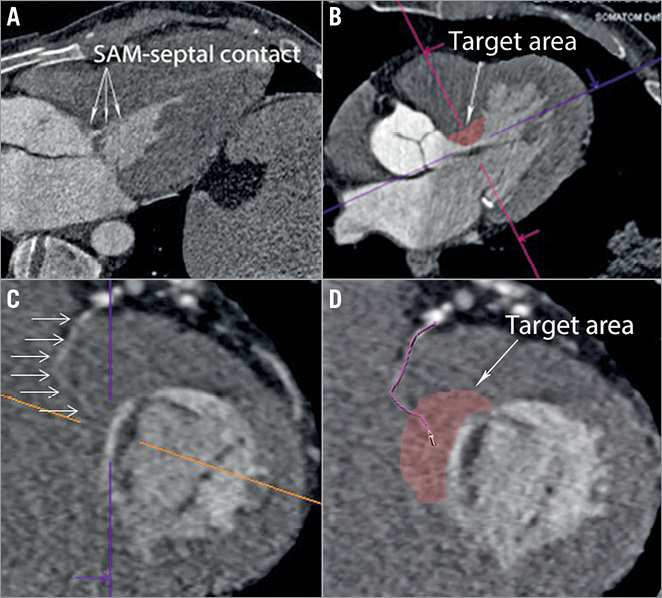
Figure 2. Septal artery tracking in 2D CT. A) Three-chamber systolic CT image displaying SAM of the MV. The contact area is seen in the basal septum. B) The target area of myocardium in the basal septum is located at the centre of the coloured lines – a short-axis view is displayed in panel C. The centre point of these lines is the same target myocardium in each image. This myocardium is then surveyed for evidence of a coronary artery; the vessel is traced back to its parent epicardial vessel and examined (D).
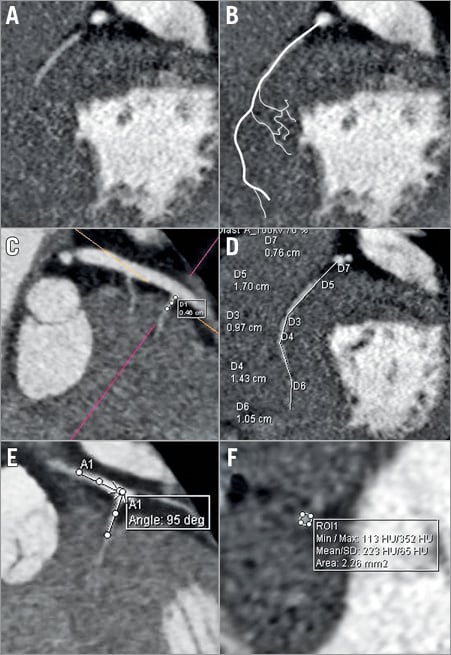
Figure 3. Anatomical details taken from 2D CT images. A) Short-axis view at the level of target myocardium. A septal artery was seen and highlighted (B). C) Septal vessel with a measurement of the distance travelled from parent vessel to entry into the myocardium. D) Total length of septal vessel from ostium. E) Angle of entry from LAD into septal. F) Area of septal vessel.
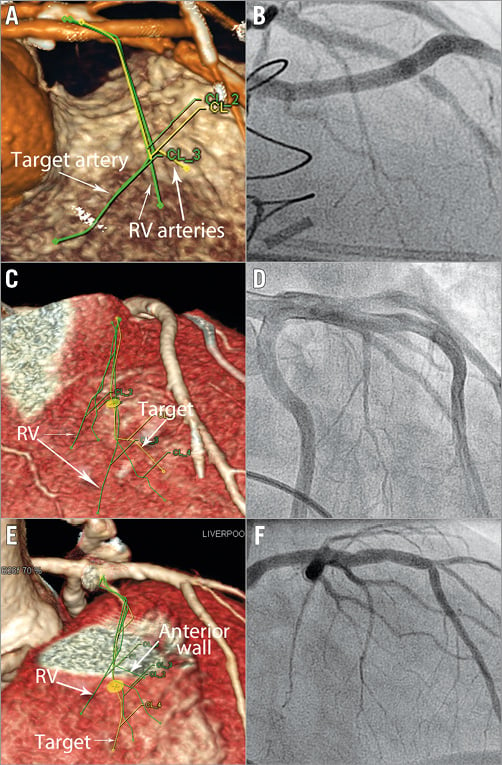
Figure 4. CT angiography with matched invasive angiography projections. A) CT angiogram. The traced septal vessels from 2D images were projected onto the coronary angiogram “map” (Moving image 1). This CT angiogram was rotated to minimise foreshortening and remove overlap (in this example to RAO cranial). The equivalent invasive angiogram projection is shown in panel B. The target artery is identified and only this sub-branch is occluded for alcohol delivery. Further examples are shown in panels C & D and E & F.
ALCOHOL SEPTAL ABLATION PROCEDURE
Patients without a permanent pacing system had a temporary pacing wire (TPW) placed in the RV apex. NBIH™ bipolar pacing wires (6 Fr) (Bard Medical, Covington, GA, USA) were used for the first 14 patients; 5 Fr Bard balloon flow-assisted bipolar wires were used after that. The working projections identified from CT to visualise the origin of the target septal from its parent vessel were implemented. A coronary wire was passed into the target septal. A traditional approach to contrast echo studies was employed first. An over-the-wire (OTW) balloon was inflated in the common ostium of the target septal with subsequent contrast injection and echocardiographic studies. OTW balloons were intentionally short; typical balloon sizes were 1.5×8 mm and 2.0×8 mm. If contrast was seen to travel to the RV septum or RV cavity (as predicted by CT), alcohol was not injected (Figure 5, Moving image 2). This was often in addition to some opacification of the LV septum. The wire was then passed in to the target sub-branch of the septal vessel. A second set of views was identified from CT to visualise the distal branch pattern. Once the wire was in the proximal portion of the vessel, the angiographic projections were changed to allow navigation into the chosen sub-branch as advised by CT (Moving image 1). When the wire was secure in the desired sub-branch, the OTW balloon was advanced and inflated to allow delivery of contrast and alcohol. If the target myocardium was not completely covered during myocardial contrast echo studies, additional target vessels were explored (n=1). The delivery of alcohol was guided by CT and confirmed by myocardial contrast studies in all patients. Other aspects of the procedure were as described previously6.
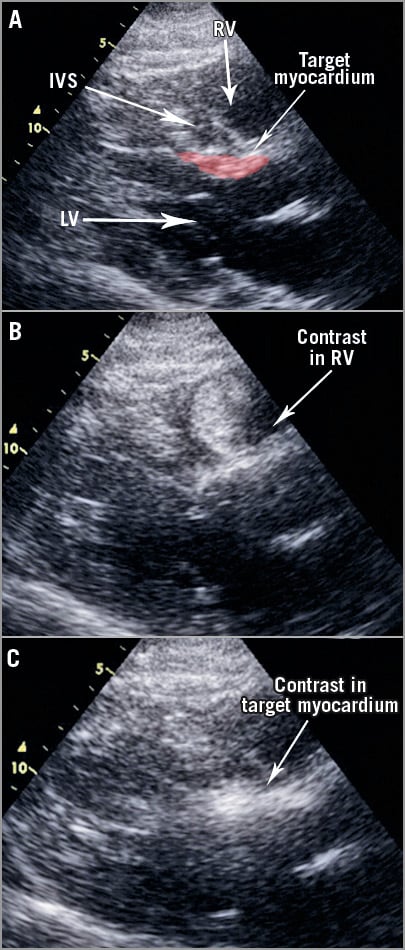
Figure 5. Myocardial contrast echocardiography studies. A) Parasternal long-axis echo view with relevant structures highlighted. Contrast injection into the common ostium of a septal; myocardial contrast is seen predominantly in the RV (B). There is some hyperenhancement in the target septum but this passes quickly (Moving image 2). C) Contrast localises to the target myocardium following occlusion of the chosen sub-branch.
STATISTICAL ANALYSIS
Continuous data are presented as means and SD, or medians and IQR for data that are non-parametric. Paired t-tests were used for parametric data and Wilcoxon signed-rank tests for non-parametric data. Change in the categorical extent of SAM2 was assessed using the sign test. Statistical analysis was performed using StatsDirect, version 2.8.0 (StatsDirect Ltd, Altrincham, Cheshire, United Kingdom).
COMPARISON TO TRADITIONAL ASA
We have reported our outcomes in 88 patients managed with conventional ASA5. Our experience mirrors international norms4,8,9. We compared the results of the CT-guided cohort against our historic controls.
Fisher’s exact test was used to compare success in treating LVOT gradients after one procedure; failure was defined as a persisting gradient of >50 mmHg or failure to reduce the gradient by greater than 50%. Fisher’s exact test was also used to compare the incidence of procedure-related complications. We compared the relationship between alcohol dose and myocardial damage as a reflection of the control of the procedure using Pearson’s correlation coefficient between alcohol dose injected and CKMB release.
Results
PROCEDURAL DETAILS
All procedures were performed by R. Stables. Twenty-one patients received alcohol to the target septal artery. One patient underwent a second ASA procedure in the study period. Alcohol was injected into two septal arteries at the same procedure in one further patient. Mean volume of alcohol delivered was 1.95 (±0.62) mL. Mean CKMB release was 116.65 (±63.30) ng/dL (reference range <5 ng/dL). A correlation between alcohol dose and CKMB release was seen; the R value was 0.46 (p=0.03) (Figure 6).
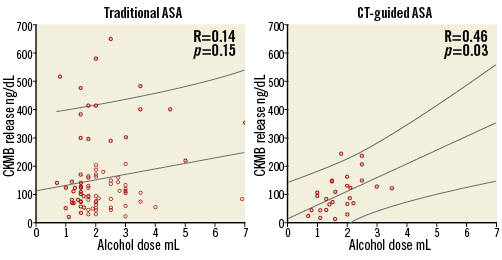
Figure 6. Relationship of alcohol dose and infarct size as assessed by CKMB release. In our historic controls treated with traditional methods there is a weak relationship; R value of 0.14 (p=0.15). In the CT-guided cohort, the relationship is much stronger; R value of 0.46 (p=0.03).
SEPTAL ARTERIAL ANATOMY IDENTIFIED BY CT
CT analysis revealed a total of 49 vessels travelling to the basal septum, an average of 2.33±1.55 septals per patient (range 1-6). Of these vessels, 24 were deemed to be to target myocardium at the SAM-septal contact point (one vessel per patient in 18, two vessels per patient in three). Eighteen of the 24 (75%) had LV and RV supply. Seven of the 24 (29%) had a third territory supplied also (anterior wall n=3, inferior wall n=2, LV mid septum n=2).
ADAPTATION TO TRADITIONAL METHODS BASED ON INFORMATION FROM CT
The information from CT altered the approach to ASA. For 17 patients, CT angiography highlighted the target for alcohol to be a specific sub-branch of a septal vessel. A sub-selective alcohol injection into the specific target branch was advised in these patients. In those septals with a branch to the RV (n=17), the initial contrast injection into the ostium of the vessel resulted in some opacification of the RV septum or cavity in all. Manipulation of the wire into the chosen sub-branch improved contrast localisation to the LV septum. In two procedures, the parent epicardial vessel for the target septal branch was the circumflex artery. For a further two procedures a small septal artery was identified from the proximal LAD.
PROCEDURAL COMPLICATIONS
Complete heart block requiring permanent pacemaker implantation was seen in 2/21 patients. Two patients developed minor pericardial effusion (<10 mm) without tamponade; no treatment was required. No late gadolinium enhancement was seen outside the target basal septum at three-day or six-month CMR scans. This was presumed to be due to perforation of the right ventricular wall by a TPW. No effusion was seen at repeat echocardiography at one month. New RBBB was seen in 2/16 (three paced prior to ASA to treat LVOT gradients, two with complete heart block [CHB] and permanent pacemaker [PPM] as a result of ASA), and new LBBB was seen in 1/16.
SURVIVAL AND RISK OF VENTRICULAR ARRHYTHMIA
Follow-up data are presented for a mean period of 375 (±137) days. All patients had assessment >180 days from ASA. One patient would not return for clinical assessment but reported improved symptoms by telephone consultation. No patient who received alcohol to the target artery died or suffered ventricular arrhythmia.
SYMPTOMATIC RESOLUTION
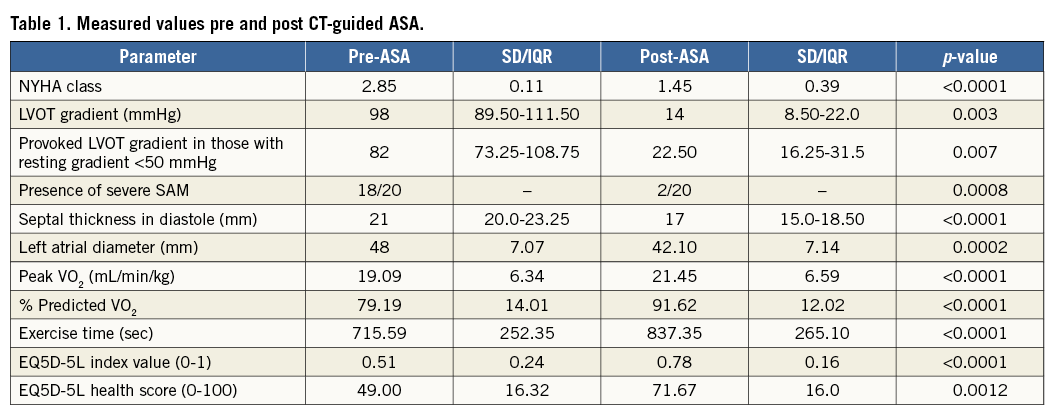
Symptoms of dyspnoea improved in 18 (90%), and mean NYHA class improved from 2.85 (±0.11) to 1.45 (±0.39) (p<0.0001) (Table 1). Ten patients improved from Class III to Class I. Five patients improved from Class III to Class II. Three patients improved from Class II to Class I and experienced resolution of recurrent pre-syncope. Two patients found no benefit and remained in Class III (one developed pulmonary fibrosis and had persistent dyspnoea despite successful gradient resolution). Chest pain was reported in 11/20 prior to ASA, all resolved with ASA (p=0.005).
ECHOCARDIOGRAPHIC DATA
Twelve of 20 patients had a resting gradient of ≥50 mmHg, and a further eight had a resting gradient of <50 mmHg and a Valsalva or exercise stress-induced gradient of ≥50 mmHg. Those with a resting gradient ≥50 mmHg improved from 98 (IQR 89.50-111.50) mmHg to 14 (IQR 8.50-22) mmHg (p=0.003). Two of 12 had a persisting gradient of ≥50 mmHg at the end of the study period. These patients had failure of resolution of LVOT gradient according to the predefined criteria detailed in the Methods section. In those with a provoked gradient, we saw an improvement from 82 (IQR 73.25-108.75) mmHg to 22 (IQR 16.25-31.50) mmHg (p=0.007). None had a provoked gradient ≥50 mmHg after ASA.
SAM improved in 18/20 patients (p=0.0008). Eighteen of 20 patients had SAM-septal contact at rest prior to ASA; 2/20 had contact after treatment. Two patients had SAM-septal contact associated with high LVOT gradients on exercise; neither had contact on exercise after ASA. Those with persisting SAM-septal contact had significant LVOT gradients.
Interventricular septal thickness in diastole decreased from 21 (IQR 20-23.25) mm to 17 (IQR 15-18.50) mm (p<0.0001). Left atrial diameter decreased from 48 (±7.07) mm to 42.10 (±7.14) mm (p=0.0002).
CARDIOPULMONARY EXERCISE TEST
Satisfactory CPEX data were available in 17 patients. Peak VO2 increased from 19.09 (±6.34) to 21.45 (±6.59) mL/min/kg (p<0.0001), representing an increase from 79.19% of predicted value (±14.01) to 91.62% predicted (±12.02) (p<0.0001). Exercise time improved from 715.59 (±252.35) seconds to 837.35 (±265.10) seconds (p<0.0001).
QUALITY OF LIFE
EQ5D-5L questionnaires were completed before and >6 months after ASA in 15 patients. The index value improved in all, with an increase from 0.51 (±0.24) to 0.78 (±0.16) (p<0.0001). The overall health score increased in 14/15 patients, values improving from 49.00 (±16.32) to 71.67 (±16.00) (p=0.0012).
COMPARISON TO TRADITIONAL ASA
Successful LVOT gradient resolution after receiving alcohol as part of a single ablation procedure was observed in 17/20 (85%) cases in the CT-guided ASA and 44/75 (59%) in the traditional methods groups (p=0.02). Those who did not receive alcohol were excluded from both the traditional methods group and the CT-guided study group.
To ensure the improvement was related to CT methods and not to the well-recognised learning curve associated with ASA, we analysed the last 20 patients treated with traditional methods. This group represented procedure numbers 105-124 performed at our centre at a rate of 10/year. This is in keeping with international guidance for adequate training10. This group had the same success at first procedure rate, i.e., 12/20 (60%) compared to 44/75 (59%) (p=0.92). When comparing the CT-guided group to the most recent traditional methods group, we see a trend towards improvement with CT, i.e., 17/20 (85%) vs. 12/20 (60%) (p=0.09). Statistical significance is lost due to smaller numbers in the sub-selected traditional group.
New RBBB was observed in 2/16 (13%) patients treated with CT-guided ASA and 42/68 (62%) patients treated with traditional methods (p=0.0004) (Table 2). CHB requiring pacemaker implantation was seen in 2/21 (10%) in the CT-guided group and 14/74 (17%) in the traditional ASA group (p=0.17).
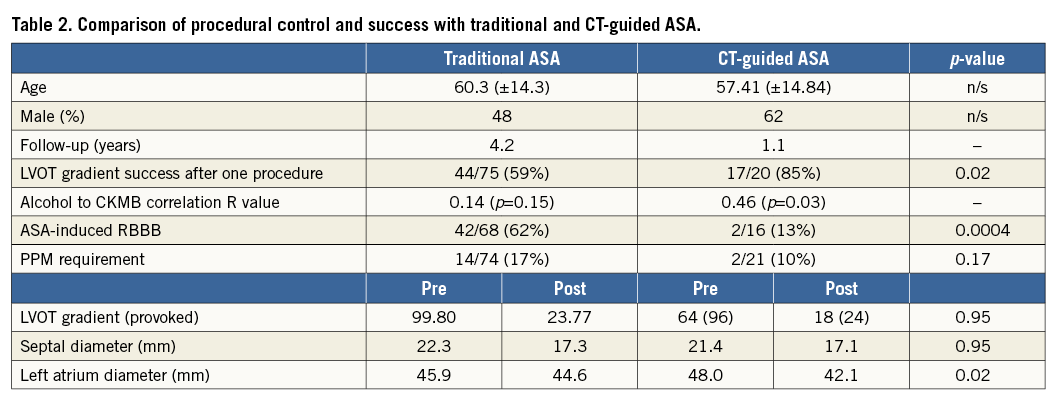
The total alcohol dose delivered was not significantly different between groups (2.24±1.08 in traditional ASA vs. 1.95±0.62 mL in CT-guided ASA [p=0.20]). Alcohol dose to CKMB release had a significant correlation in the CT-guided group (R value 0.46, p=0.03), whereas no significant correlation was seen in the traditional group (R value 0.15, p=0.14) (Figure 6).
Symptom improvement, defined by change in NYHA class, was seen in 18/20 (90%) patients with CT-guided ASA and 55/75 (73%) patients treated with traditional methods (p=0.12). Direct comparisons are difficult as patients in the traditional group received a greater number of doses of alcohol, 1.23 (±0.48) vs. 1.05 (±0.22) in the CT-guided group (p=0.17).
PATIENTS UNABLE TO RECEIVE ALCOHOL AND INTENTION-TO-TREAT
Five patients did not receive alcohol (Figure 1). In two patients we could not safely access the septal artery predicted to supply the target area by CT. The coronary wire had to negotiate many turns, and when the OTW balloon was advanced the wire prolapsed back into the parent epicardial artery. These patients were forwarded for an alternative form of septal reduction therapy (radiofrequency ablation in three, myectomy in one). One patient died three months later following a stroke whilst awaiting a further treatment decision. These patients are not included in the outcome analysis as they did not have repeat assessment including echocardiography and CPEX prior to further treatment.
Four of five patients were included in an intention-to-treat analysis (myectomy patient excluded). This used the pre-assessment echocardiographic data as the post-assessment value as no treatment was delivered (no repeat data were available prior to completing the next treatment modality for LVOT obstruction). Using this method, the resting LVOT gradient improved from 64 (IQR 28-100), provoked gradient 96 (IQR 78-120) to 23 (IQR 9-30), provoked 32 (IQR 14-39), respectively (p<0.0001). The success rate following one procedure was then 71% (17/24). Comparison to a traditional group is difficult as the indications to enter the lab for ASA have evolved. Including all patients who entered the lab for ASA, the success rate of the traditional group after first procedure was 51% (44/86) (p=0.09). This type of analysis holds many unavoidable limitations.
Discussion
CT angiography can provide high-quality images of septal coronary arteries. The images provide anatomic insights that have altered our approach to alcohol delivery in ASA.
A septal artery can often be seen on CT to supply both the RV septum and the LV septum, which are very different environments. This has implications for variable “run-off”. Any fluid injected into the proximal portion of this artery will follow the path of least resistance. This means that contrast (or alcohol) will flow into the low-pressure circuit of the RV septum and drain directly into the RV cavity via Thebesian veins, rather than enter the high-pressure circuit of the densely packed hypertrophied LV septum. Traditional teaching for alcohol ablation is to site a coronary balloon at the ostium of the septal artery, and inject beyond11,12. This would result in echocardiographic contrast highlighting the RV; this pattern of myocardial contrast has been described as the most common undesirable location. The vessel would then be dismissed as serving an incorrect area of myocardium. Engaging the sub-branch of the septal vessel that supplies the LV myocardium can result in target myocardium being highlighted (Figure 6, Moving image 2). An artery that would previously have been dismissed now becomes an ideal target.
The variable branching pattern involving right and left ventricular myocardium also has an effect on the ability of injected alcohol to damage myocardium. The differing and unpredictable coronary pressures affect vascular resistance, the route and rate of venous drainage and hence the “dwell time” of alcohol in the myocardium. This was reflected in the poor correlation between alcohol dose and CKMB release in our traditional ASA group. This is much improved when injecting into selective LV branches identified by CT, with a more predictable amount of damage observed.
The parent vessel for the appropriate septal artery is not always the LAD. Patients have been identified with basal septum supply from the circumflex and intermediate arteries11 and right coronary artery. In 5/59 patients in one series, the target vessel did not originate from the LAD. These patients may be dismissed as not having an appropriate artery by operators who may not consider other parent arteries13. The approach to identifying the correct artery is reversed in CT. In traditional ASA, we follow a vessel from its origins. Using CT, we identify the appropriate vessel for alcohol delivery in the target myocardium and track it back to its source, wherever that parent artery may be. This will avoid missing vessels due to unexpected origins.
Myocardial contrast echocardiography (MCE) remains a prerequisite in ASA; CT and MCE are to be used as complementary modalities. In this study, MCE was used as a proof of concept to display vascular supply to both the LV septum and the RV septum in those predicted to have dual supply, prior to engaging our true target sub-branch.
CT-GUIDED APPROACH IMPROVES CONTROL OF INFARCT SIZE AND LOCATION
CT-guided ASA may afford greater control of the size and location of infarct. The increased control is suggested by the better correlation of alcohol dose to CKMB release. Previously, it was difficult to predict the extent of myocardial damage, in part due to the inability to control variable run-off into different myocardial territories. We can now predict the extent of myocardial damage as indicated by CKMB release.
RBBB was a very common procedural observation using traditional methods8,14,15. RBBB has even been suggested as a predictor of good outcome from ASA15. Whilst this association is seen, it is probably explained by the presence of transmural infarcts that incorporate left and right ventricular myocardium14. Infarction of the right bundle is collateral damage and should not be the primary target. The true target is the left ventricular myocardium, aiming to reduce size and systolic excursion into the LVOT. Both changes have an effect on LV haemodynamics. It is not simply reduction in the size of the septum we seek, as gradients can be reduced significantly with minimal change. This is observed in radiofrequency ablation of the septum16. The reduction in systolic excursion of the septum and hence SAM is responsible for this reduction in gradients.
The significant reduction in RBBB in CT-guided ASA is indicative of more targeted infarct in the LV myocardium. This has been confirmed by CMR studies. Seven of nine patients who were able to undergo CMR post ASA showed RV endocardial sparing (Figure 7). We were unable to perform CMR in 11 (ICD/PPM in nine, claustrophobia in two). CT allows us to target the appropriate LV myocardium with greater accuracy.
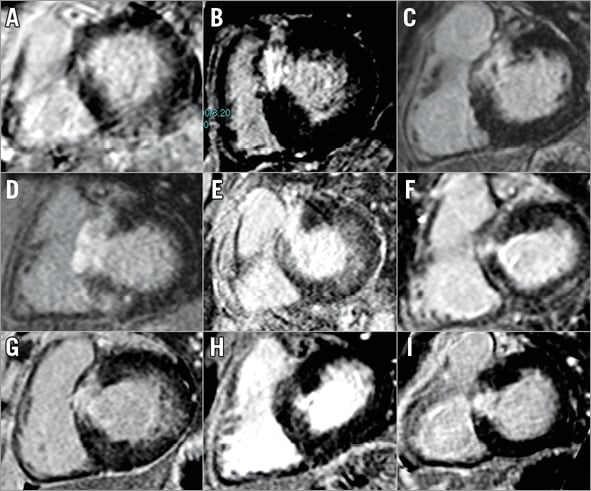
Figure 7. Post-ASA late gadolinium CMR imaging. Nine patients underwent CMR scanning six months post ASA. Panels A to I display late gadolinium enhancement short-axis images one slice below the LVOT; this represents the target myocardium.
EFFECT OF BETTER INFARCT LOCALISATION ON CLINICAL OUTCOMES
A more accurate infarct will create a more favourable effect on LVOT haemodynamics. Mean gradients and symptom burden were much improved after CT-guided ASA. This has been true for most case series reported historically. The average gradient in the CT group is composed of a greater proportion of successful procedures than in our traditional group. This is despite there being a reduced need for additional procedures to reach this outcome. This greater procedural success rate results in an improved exercise capacity and a trend towards a greater proportion of patients reporting a symptomatic improvement. This does not reach statistical significance compared to the effect of a traditional approach in this relatively small group of patients.
RADIATION RISKS
The mean radiation dose per CT scan was 3.28 mSv. A procedure with this dose is classed as low risk with a <1 in 10,000 additional risk of fatal cancer in an adult17. A standard angiogram with 4.3 minutes screening time and seven diagnostic shots uses 3.9-4.9 mSv (this is the UK average)17. The mean radiation dose for ASA was 9.04 mSv (3.84-34.08 mSv). The use of CT is leading to more effective treatment at the first attempt (p=0.02). These adaptations will ultimately reduce the delivered radiation to the population and compensate for the small dose from CT.
Conclusions
CT angiography has changed our approach to ASA. This refined approach improves control and location of iatrogenic infarct. This is translating into greater success in treating LVOT gradients at the first procedure and has potential to have a greater impact on patients’ symptoms.
| Impact on daily practice A substantial proportion of patients undergoing alcohol septal ablation for obstruction in hypertrophic cardiomyopathy have significant persisting LVOT gradients, often due to inaccurate location of the iatrogenic infarct. CT angiography planning with alcohol injection into pre-identified sub-branches of septal arteries improves localisation of infarct. This improved accuracy leads to a greater success in treating LVOT gradients at first procedure and is translating into improved symptoms and functional status. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. The process of CT identification of the septal artery and subsequent ASA procedure. The initial image shows the SAM-septal contact area on the septum. This frame is in systole. The next image shows the same myocardium in the basal septum in diastole. The three-chamber diastolic image is part of a four-panel relational CT image displayed in the syngo.via workstation. The central point of the cross-sectional lines is the same in each of the three 2D images. A septal vessel is seen tracking into the target myocardium in the short-axis view when the image is zoomed in (labelled by white arrows). This vessel is then tracked, and the line created when tracking this target vessel is then transferred to a 3D rotational angiogram. The familiar major epicardial vessels are seen in the traditional shade of orange. The target septal vessel is represented by the yellow line, and other branches of the same vessel which travel to territories that we do not wish to infarct are represented by the green lines. This angiogram is then rotated to display the septal artery in a range of projections that are possible in the catherisation lab. The aim is to choose familiar views that remove foreshortening and overlap, and display the fine detail of the vessel to allow the operator to navigate a wire to the target sub-branch. We often choose one projection to enter the mouth of the septal vessel, and another to expose the necessary detail to pass to the distal target branch. Examples of the images created by CT with invasive angiograms are shown in RAO cranial, plain AP and LAO caudal. In the last example, the LAO caudal view was used to pass the over-the-wire balloon to the correct sub-branch. Myocardial contrast was delivered after balloon occlusion, localising to the target myocardium on echocardiography. Alcohol was delivered; the subsequent angiogram shows no reflow in the target sub-branch. Echocardiography at six months shows resolution of gradient with good localisation of infarct in the basal septum. SAM has resolved.
Moving image 2. Myocardial contrast echocardiography with traditional balloon occlusion in the mouth of the septal vessel and then on selective sub-branch occlusion. On-table transthoracic echocardiography. The first contrast injection is seen in the parasternal long-axis view. Contrast was injected into the common stem of the septal vessel as per traditional methods. This briefly localises to the basal septum and then washes out into the right ventricle. In the second injection, the balloon has been advanced into the target sub-branch, selective injection localises to the target septum only and has a long “dwell time” in the myocardium.
Supplementary data
To read the full content of this article, please download the PDF.
The process of CT identification of the septal artery and subsequent ASA procedure.
Myocardial contrast echocardiography with traditional balloon occlusion in the mouth of the septal vessel and then on selective sub-branch occlusion.

