Abstract
Aims: The best strategy for stenting in bifurcations remains unclear. Szabo et al described a technique for accurate stent placement in bifurcations 010-001 or in aorto-ostial lesions. Its feasibility has been validated in animal models and small clinical series, but its safety and procedural results have never been compared to conventional positioning.
Methods and results: In a retrospective search, 257 out of 2,596 intervened lesions corresponded to Medina 010 (108, 42.0%)/001 (66, 25.7%) bifurcations or aorto-ostial lesions (83, 32.3%). Szabo was the initial choice in 78. Crude analysis showed significant differences between groups in several control variables, that disappeared after propensity score matching. Cross-over occurred in nine (11.7%) Szabo cases vs. no case in the conventional group. Two independent blinded investigators evaluated the angiographic result immediately after stent deployment. Szabo reduced the incidence of stent malpositioning (6.4% vs. 41.0%, p=0.000001), protrusion in the non-stented vessel/aorta (6.4% vs. 34.6%, p=0.00003) and incomplete scaffolding of the plaque (0.0% vs. 7.7%, p=NA). No significant differences regarding complications, procedural success or procedural complexity were observed after 30 days follow-up.
Conclusions: The Szabo technique reduces the incidence of angiographic malpositioning in Medina 010/001 bifurcations and aorto-ostial lesions, without increasing procedural complications.
Introduction
Since the era of plain balloon angioplasty, percutaneous coronary intervention (PCI) of bifurcation lesions has been technically challenging and associated to higher rates of procedural complications and worse outcomes1-4. Stenting minimised the incidence of acute vessel closure and procedural complications, but higher rates of restenosis still remained an important drawback5-8. The use of drug-eluting stents (DES) improved substantially the rates of restenosis and major adverse cardiovascular events (MACE)9-14. Thus, the ARTS II study did not find significant differences in MACE at one year follow-up between bifurcations and non-bifurcation lesions treated with DES15, but the former were associated to more complex and prolonged procedures. Several observational studies and randomised trials have found that a simple provisional stenting strategy reduces the procedural complexity and has angiographic and clinical results at least as good as a complex 2-stents strategy7,10,16-19. Thus, provisional stenting is currently accepted as the standard approach for PCI of bifurcation lesions. However, the high cross-over10 or a possible selection bias16-19 suggest that this approach is not always feasible or might not be the best choice for many cases. Therefore the best stenting strategy for bifurcation lesions is still a matter of dispute20.
Bifurcation types 010 and 001 of Medina21 are a subgroup where the best PCI strategy has not been very well defined yet. The SCANDSTENT11,12 or NORDIC trials16 do not report their results according to Medina classification, but in other randomised trials these account for less than 1%9 or up to 18,318 of the total bifurcation PCIs. Szabo and Kern have recently described a smart technique for accurate stent placement in bifurcation types 001 and 010 of Medina where the initial intention is ostial stenting of the side branch or the distal main vessel, respectively22. The technique could ease the accurate deployment of the stent at the carina, preventing excessive forward and insufficient progression alike. An excessive forward progression of the stent could entail suboptimal scaffolding of the plaque and hence increase the risk of restenosis10,19, whereas an insufficient progression could entail protrusion of some struts into the bifurcation core (floating struts), undergoing an irregular process of neointimal coverage and eventually increasing the risk of thrombotic complications. The technique is also suitable for aorto-ostial lesions.
The Szabo technique has been validated in animal models22 and small clinical series of selected cases22,23, reporting good angiographic and IVUS results. Nevertheless, to our knowledge it has been never compared to the conventional angiographic placement in larger clinical series of unselected cases hitherto, and no comparative follow-up results have been reported. Therefore, it is uncertain whether the Szabo technique is more accurate than simple angiographic positioning of the stent, and whether this accuracy results in better clinical outcomes. Moreover, the Szabo technique is sensibly more complex, skill-demanding and associated to some specific procedural complications, whose incidence in series of non-selected cases is also unknown.
Materials and methods
The study followed a retrospective non-population cohort design, based on a search in the database of the CathLab in Meixoeiro Hospital (Vigo, Spain), screening all the PCIs performed between May 5, 2007 (date of the first local Szabo procedure) and October 22, 2008. The inclusion criteria were PCIs involving: 1) bifurcation types 001 or 010 of Medina and 2) aorto-ostial lesion. Ostial lesions in the left descending anterior (LAD) and the Circumflex (LCX) were considered bifurcation types 010 and 001, respectively. Exclusion criteria were: 1) Initial intention of 1.a) treating both branches of the bifurcation, 1.b) using a 2-stents technique, or 1.c) jailing a vessel (main vessel [MV] across side branch [SB] or MV to SB techniques, according to EBC consensus20); 2) Need to advance the stent through the struts of a previously implanted stent; 3) PCI on coronary artery bypass graft (CABG); 4) Excessive vessel tortuosity, precluding the choice for Szabo; 5) Known clinical condition conferring a life expectancy <12 months at the time of the intervention. An intervention cohort and a control cohort were defined according to the technique employed (Szabo or conventional angiography, respectively).
Objectives and control variables
Angiographic malpositioning of the stent was the primary objective, and defined as angiographic evidence of the implanted stent protruding into the non-stented branch (or into the aorta) or of incomplete plaque scaffolding. The baseline angiography of the target lesion and the immediate-post-deployment angiographic result were recorded, avoiding sequences of the PCI procedure. In 71 lesions of the Szabo group (91.0%) and in 68 of the angiography group (87.2%) the final result included a recording using Stent Boost Enhancement (Philips Medical Systems, Eindhoven, The Netherlands). Two independent external investigators, blinded to the employed technique, evaluated offline the angiographic result, assisted by bifurcation-dedicated QCA software (PIE Medical Imaging, Maastricht, The Netherlands). The stent was considered to protrude into the non-stented vessel if the latter: 1) presented final TIMI flow lower than initial TIMI flow, 2) presented final calibre smaller than initial calibre, 3) was jailed, 4) floating struts could be identified in the bifurcation core. Likewise, angiographic evidence of incomplete plaque scaffolding was defined as residual stenosis ≥20% in the stented segment by QCA, namely segment 8 (side branch) in 001 lesions or segment 3 (distal main vessel) in 010 lesions (Figure 1). For the evaluation of the results in aorto-ostial lesions no objective criteria could be defined, and the decision depended on the subjective appreciation of two independent cardiologists. In the case of discrepancy between the two investigators, a third investigator acted as referee.
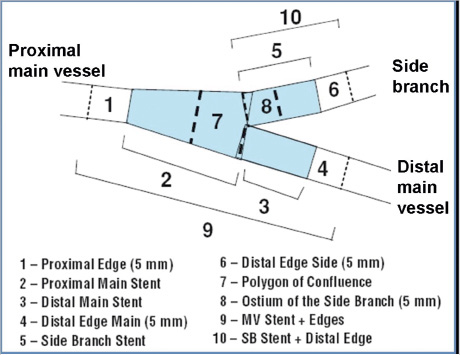
Figure 1. 10-segments-model in bifurcation-dedicated QCA (PIE Medical). A residual stenosis ≥20% in the segment 8 or in the segment 3 was the objective criterion for incomplete plaque scaffolding in lesions 001 or 010, respectively.
Secondary objectives were angiographic success, procedural outcomes and procedural complexity. Angiographic success was defined as final TIMI 3 flow with a final residual stenosis <20% in both branches of the bifurcation. The procedural outcomes comprised periprocedural complications (death, myocardial infarction [MI] or stroke) up to 24 hours after the intervention, major adverse cardiovascular events (MACE) at 30 days follow-up or during index hospitalisation and procedural success, defined as angiographic success without MACE at 30 days. MACE was the composite of death, MI, stroke or need for revascularisation. Postprocedural MI was defined as a rise in serum troponins above three times the upper limit of normality24. Procedural complexity was estimated through the amount of contrast and radiation, the procedure time, and the incidence of procedural problems. Procedural problem was defined as any unexpected event that prolongs the procedure, increases the risk of complications, or requires bail-out techniques or devices.
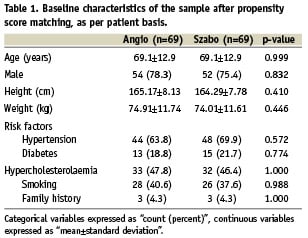
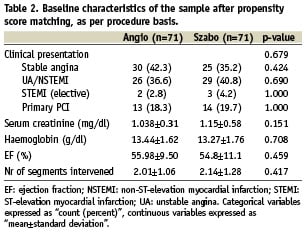
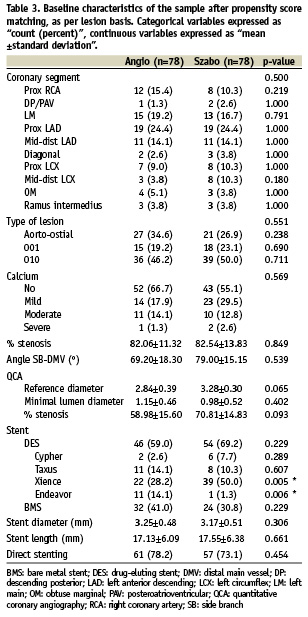
Patient characteristics, cardiovascular risk factors, clinical indication, pre-procedural medication and several clinical and procedural variables were controlled in order to guarantee the comparability of the groups (Tables 1-3).
A subgroup analysis of the primary objective according to the type of lesion (bifurcation Medina 010, 001 or aorto-ostial lesion) was planned.
Description of the intervention
In the intervention cohort, the Szabo technique was performed by placing a wire in the target vessel, and a second wire (tail-anchor wire) in the non-stented vessel or floating in the aortic bulb in the case of aorto-ostial lesions. In most cases, BMW wires (Abbott Vascular, Santa Clara, CA, USA) were employed. The stent was then loaded onto the guidewire in the target vessel, and the proximal tip of the tail-anchor wire was carefully threaded through the last cell of the stent, on a direct way. The stent was then advanced over both wires, until the system reached the carina of the bifurcation or the ostium of the vessel. At this point the tail-wire anchored the proximal edge of the stent precisely at the carina or at the coronary ostium (Figure 2). The stent balloon was then inflated at 6 atm pressure, the tail-anchor wire removed, and the inflation completed to full pressure.
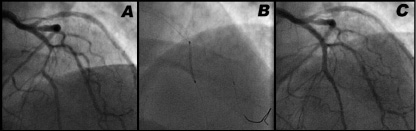
Figure 2. Example of the Szabo technique for stenting a bifurcation type 010 of Medina. Severe stenosis in mid LAD (distal main vessel), immediately distal to the take-off of the first diagonal branch (side branch) (A). The Szabo technique allows the stent to advance through the LAD until the tail-wire anchors the proximal end exactly at the carina (B). At this point the stent can be deployed, with a nice final result (C).
In the control cohort, stent was placed under fluoroscopic and angiographic guidance.
During the study period, the standard practice in the lab for both the intervention and the control group was performing kissing balloon in case of TIMI flow <3 or residual stenosis >70% by visual estimation in the non-stented vessel.
Statistical analysis
The intervention cohort and the control cohort were analysed according to the principle of intention-to-treat, considering the initial technique chosen by the operator.
Crude analysis compared baseline characteristics and result variables. Saphiro-Wilk normality test was performed on continuous variables. If a normal distribution could be assumed they were compared with t-test for independent samples; otherwise comparison was done with U-Mann-Whitney test. Categorical variables were compared with Pearson chi-square, or with Fisher’s exact test if the expected count was <5 in any cell.
Due to significant differences in baseline characteristics between the groups, affecting their comparability, propensity-score matching was performed, using a logistic regression model that included all the variables with p<0.2 in the crude analysis, following the method of nearest neighbour without calliper. After matching, continuous variables were compared with t-test for paired samples, while categorical variables were compared with McNemar test.
For the analysis of subgroups a pooled analysis method was employed, with the relative risk as summary parameter, following a fixed effects model, weighting by the inverse of the variance, and proportional correction for zero effects.
The statistical analysis was performed using the program SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
During the study period, 2,596 coronary lesions underwent PCI (2,283 procedures, 1,991 patients). Of these, 268 lesions met inclusion criteria, 11 lesions were excluded due to initial intention of “MV across SB” (three subjects), initial intention of “MV to SB” (one subject), need to advance the stent through the struts of a previously implanted stent (six subjects) or to CABG PCI (one subject). Two hundred and fifty-seven lesions (9.9%) were finally eligible (219 procedures, 213 patients), corresponding to ostial lesions (83; 32.3%), 010 bifurcations (108; 42%) or 001 bifurcations (66; 25.7%). Szabo was the operator’s first choice in 78 lesions (71 procedures, 69 patients), while conventional angiographic guidance was the first choice in 179 lesions (148 procedures, 144 patients) (Figure 3). Both techniques were concurrent in time. Of these procedures, 90.4% were performed through the radial approach, and 98,7% using a 6 Fr guiding catheter.
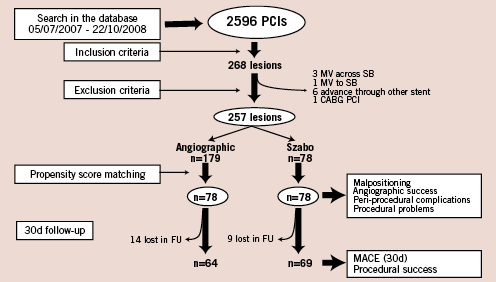
Figure 3. Flow-chart showing the formation of the cohorts, and the loss in follow-up. FU: follow-up; MACE: major acute coronary events; MV: main vessel; PCI: percutaneous coronary intervention; SB: side branch.
Crude analysis and propensity score matching
In the crude analysis, significant differences between groups were found regarding prevalence of diabetes, use of nitrates, number of lesions intervened in the procedure and direct stenting. It seemed also to be some imbalance in the anatomical distribution of the treated coronary segments, although the difference was not strictly significant. In order to permit the comparison between groups, propensity score matching was performed. The propensity regression model included all the variables were a p<0.2 in the crude analysis.
Tables 1-3 show the basal characteristics of the groups after propensity score matching, with no significant differences between groups at any of the levels of comparison.
The type of stent was not included in the propensity score, even though there were significant differences between groups, because it was considered to be strongly dependant on the choice for the type of technique, since not all types of stents are equally suitable for the Szabo technique.
Malpositioning
In the matched cohort, as per lesion analysis, the overall incidence of angiographic malpositioning was 6.4% in the Szabo group vs. 41.0% in the control group (p<0.001). In the Szabo group, all the cases of angiographic malpositioning were due to protrusion in the non-stented vessel/aorta, with no case of incomplete scaffolding of the plaque (Table 4, Figure 4), while in the angiography group protrusion was present in 34.6% of the cases (p<0.001) and incomplete scaffolding in 7.7% (McNemar test not applicable). One case in the angiography group met objective criteria for both protrusion and incomplete scaffolding, due to the small angle for the take-off of the side branch.
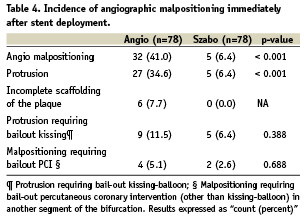
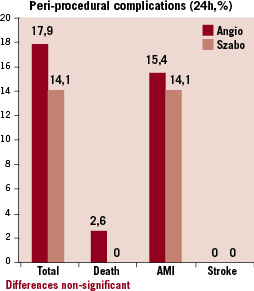
Figure 4. Incidence of malpositioning immediately after stent deployment in the intervention (Szabo) and control (angio) groups.
The incidence of protrusion requiring bail-out kissing-balloon and malpositioning requiring PCI (other than kissing-balloon) in another segment of the bifurcation tended to be lower in the Szabo than in the angio group, although not statistically significant.
In the analysis of angiographic malpositioning by type of lesion (001, 001 or aorto-ostial), no heterogeneity in the result was observed between different subgroups, (I2=0.00).
Procedural outcomes
No differences were found in angiographic success rate, periprocedural complications or acute procedural success at 24 hours. Follow-up at 30 days could be completed in 69 patients in the Szabo group (88.5%) and 64 patients in the angiographic group (82.1%), without finding significant differences between the groups in MACE or in procedural success (Table 5, Figure 5).
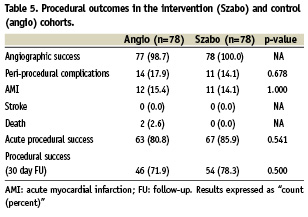
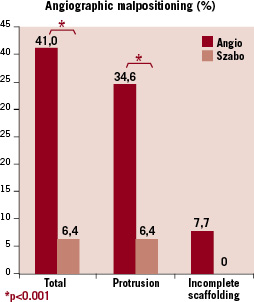
Figure 5. Incidence of procedural complications in the intervention (Szabo) and control (angio) groups.
Procedural complexity
When Szabo was the first choice, cross-over to angiographic positioning happened in 11.5% of the cases vs. no case in the angio group (McNemar not applicable).
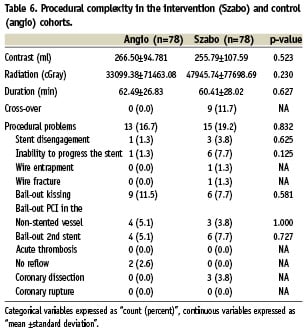
According to the intention-to-treat principle, no differences were found in contrast volume, radiation, procedure time or procedural problems (Table 6). In one Szabo case, the tail-wire got trapped into the stent struts when it was pulled out, with all attempts to liberate the wire being unsuccessful, and finally leading to fracture of the distal tip. The patient was doing well at 30 days follow-up.
Discussion
Although the Szabo technique has been rapidly accepted by and incorporated to the repertoire of the interventional cardiologists, a question has remained unanswered: does it add any real advantage to the already existing conventional angiographic method for ostial stenting? The first studies published had focused on the validation of the concept in animal models 22 and on proving its feasibility and safety in selected cases22,23, but they were not designed to compare the performance of Szabo technique versus the conventional method. To our knowledge this is the first study comparing both techniques in a real-world non-selected cohort of patients. The results confirm the hypothesis that Szabo technique is more accurate than the conventional technique in avoiding malpositioning of the stent. The incidence of stent protrusion in the non-stented vessel or in the aorta is substantially reduced, while the incomplete scaffolding of the plaque simply disappears by using Szabo. The advantage of Szabo seems to be present in all the three types of lesions considered (bifurcations 001, 010 or aorto-ostial lesions), although the most solid and definitive results are observed in bifurcations 010 of Medina, that accounted for 48.1% of the matched cohort. The mechanism underlying this extra accuracy might be double: first, a clear mechanical reference for stent placement, overcoming angiographic foreshortening; but also the reduction of systo-diastolic oscillation owed to a push-and-keep manoeuvre preceding the deployment.
Once proved that Szabo technique’s acuity is superior, the question arises if this accuracy will be translated into any clinical benefit for the patient. Our study only shows that the intervention is safe, with no differences in procedural complications or in MACE at 30 days, albeit with a non-significant difference favouring Szabo. However more prolonged follow-up is required to compare clinical outcomes.
A second question arises regarding the procedural complexity, namely the price that the interventional cardiologist has to pay for that improved accuracy in positioning. Certainly the Szabo technique is more challenging and skill-demanding than the usual procedures. Its detractors actually state that it makes little sense to go for a complex procedure, taking the risk of eventual complications, when no clear advantage has been proven. Nonetheless, in our study neither more complications nor more procedural complexity were associated with Szabo, even though the learning-curve was comprised into the study period. The procedures were not prolonged, and conversely a non-significant trend to use less contrast was observed. The trend to use more radiation might be partially explained by the academic interest of the operators in documenting (and therefore filming) extensively the novel technique. The incidence of procedural problems was not statistically significant either. However, a deeper insight into these results points out some issues that the operator should be aware of. Firstly, the cross-over rate is as high as 11.7%, mainly due to inability to advance the stent, probably caused by the twisting of the wires. A careful check of the wires should be encouraged before attempting Szabo, and if twisting is suspected, one of them should be repositioned. Secondly, stent disengagement from the delivery system, although not statistically significant in our cohort, deserves some attention, being the first problem that most operators face in the learning curve. It could be prevented by threading the proximal tip of the tail wire through the last cell of the strut on a direct way, without prior balloon inflations that could impair the crimp of the stent on the balloon, by a proper pre-dilatation, by a good catheter support and by keeping the wires always parallel along the way to the lesion inside the catheter or the coronary artery. Thirdly, the case of wire entrapment and final rupture of the distal tip is particularly worrisome due to its potential complications. To avoid this problem, some groups suggest deflating the balloon before removing the anchor-wire, and once removed completing the inflation. No balloon puncture was registered, even after threading the wire directly through the crimped stent cell.
From a merely descriptive point of view, our cohort also demonstrates that Szabo is feasible for a radial approach, using a 6 Fr catheter and direct stenting (73.1% of the intervention group), with a minimal incidence of complications, although perhaps the choice of wider guiding-catheters or systematic predilatation could have reduced the incidence of stent disengagement or the cross-over rate. Another interesting observation is that the technique strongly influences the choice of the stent. Thus, the preference is for stents whose structure and crimp to the balloon is best preserved when threading the tail-wire, rather than those stents that peel-off entirely when a cell is detached. Because of this strong interdependence, we decided not to match the cohort by the type of stent, since the rates of drug-eluting/bare-metal were comparable in both groups.
Limitations
The observational design of this study must lead to cautious interpretation of the results, even though the methodology employed (propensity score matching) warrants the best comparability possible between groups, close to the one provided by a randomised trial. It is usually recommended to perform the propensity score matching only if the ratio (control group/ index group) is ≥10. If this condition is not met, the matching might not work as expected. In our cohort the ratio is around three, but despite of this, the matching worked out fine (Tables 1-3): only five pairs had ∂>0.2 and seven pairs had ∂>0.1. The effect of the operator could not be included in the propensity score, constituting the main limitation of the study, thus the differences observed between groups could be partially due to the effect of different operators rather than to the technique itself. Due also to the retrospective nature of this study, some potential caveats of the Szabo technique could have been ignored, for instance the incidence of twisting of the wires, resulting in need for rewiring or repositioning. It is a minor but common problem in Szabo, usually not reported by the operator.
The primary result (malpositioning) is difficult to assess angiographically, even with objective criteria based on bifurcation-dedicated QCA software and the assistance of stent-boost enhancement. Certainly intravascular ultrasound, or even better optical coherence tomography, would have been able to assess this result more accurately, but they were not available in most of the patients for a retrospective comparison. Angiography was, hence, the only tool available to build a large matched cohort and compare the results.
Conclusions
The Szabo technique reduces the incidence of angiographic malpositioning compared to conventional angiographic placement in bifurcation types 010 and 001 of Medina, and in aorto-ostial lesions, without increasing procedural complications or procedural complexity.

